First-Line Nivolumab Plus Cabozantinib Improves Outcomes vs Sunitinib in Advanced Renal Cell Carcinoma
The combination of nivolumab plus cabozantinib was found to be superior to the former standard, sunitinib, in the first-line treatment of advanced or metastatic renal cell carcinoma, according to the results of the phase III CheckMate 9ER trial reported at the European Society for Medical Oncology...
Is ctDNA Effective in Detecting Genomic Alterations in Patients With Metastatic Kidney Cancer?
Circulating tumor DNA (ctDNA) analysis is a minimally invasive genomic assessment tool utilizing targeted next-generation sequencing of peripheral blood. At the ESMO Virtual Congress 2020, Zengin et al reported genomic results from a large cohort of patients with metastatic renal cell carcinoma...
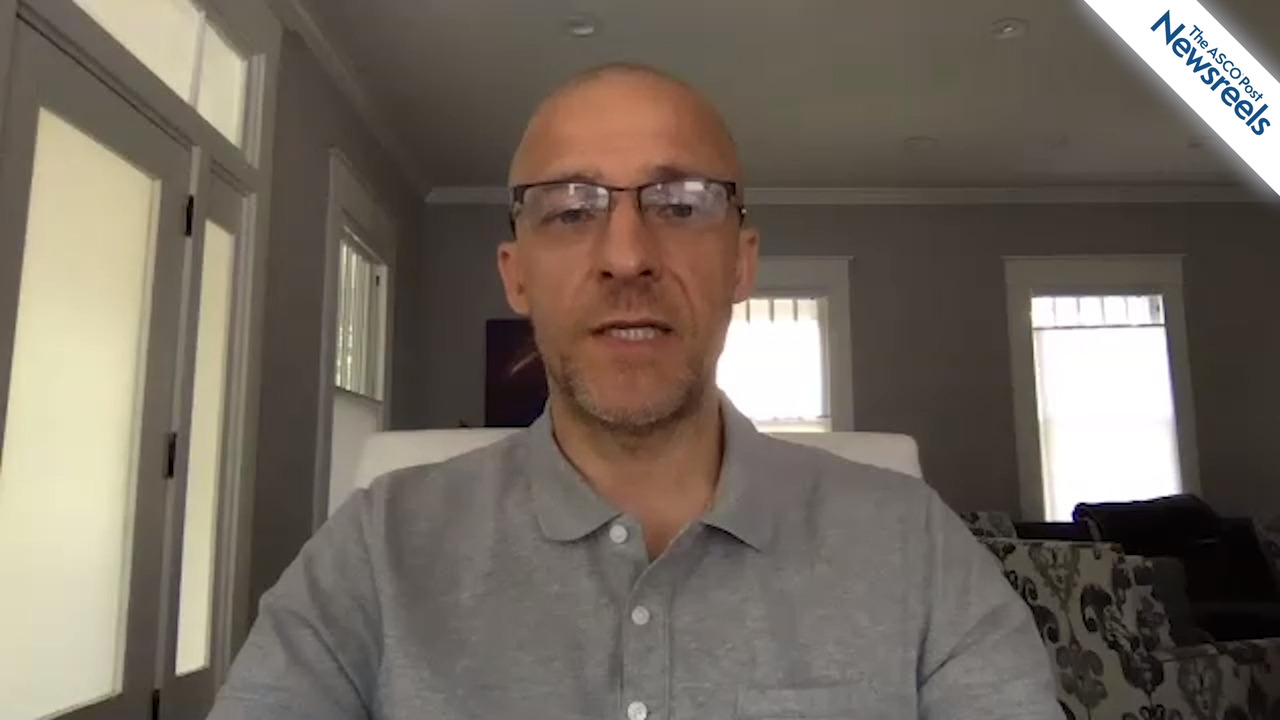
Sumanta K. Pal, MD, on Renal Cell Carcinoma: Cabozantinib Plus Atezolizumab as First-Line Therapy
Sumanta K. Pal, MD, of the City of Hope National Medical Center, discusses results from the COSMIC-021 study, which tested two different doses of cabozantinib, each with a standard dose of atezolizumab, administered to patients with metastatic advanced clear cell renal cell carcinoma. Dr. Pal reports on response rates and progression-free survival, as well as biologic correlates that may have influenced response (Abstract 702O).
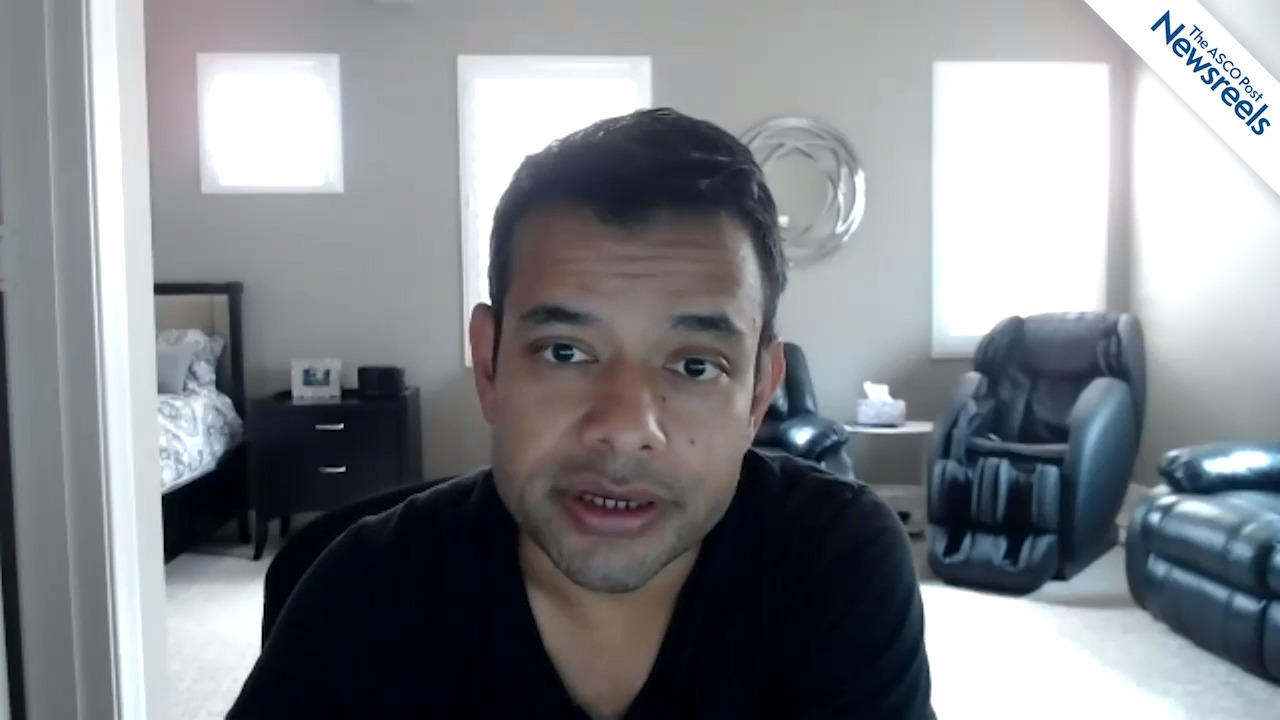
Toni K. Choueiri, MD, on RCC: Nivolumab Plus Cabozantinib vs Sunitinib in First-Line Treatment
Toni K. Choueiri, MD, of Dana-Farber Cancer Institute, discusses the first results from the phase III CheckMate 9ER trial, which suggested the combination of nivolumab and cabozantinib is safe. It showed activity in progression-free and overall survival, as well as in overall response rates and may have a place in treating patients with metastatic renal cell carcinoma (Abstract 696O_PR).
CheckMate 9ER Trial Shows Benefit of Novel Doublet for Advanced Kidney Cancer
The combination of nivolumab plus cabozantinib was superior to the former standard, sunitinib, as first-line treatment for advanced/metastatic renal cell carcinoma, according to results of the phase III CheckMate 9ER trial reported by Toni K. Choueiri, MD, and colleagues at the ESMO Virtual...
Link Between Gut Microbiome and Treatment Outcomes in Patients With Metastatic Kidney Cancer
Researchers have found that greater gut microbial diversity in patients with metastatic kidney cancer is associated with better treatment outcomes in those receiving immunotherapy. These findings were published by Salgia et al in European Urology. “We also reported the changes over time in the gut...
Study Supports Pembrolizumab Plus Axitinib in Previously Untreated Advanced Renal Cell Carcinoma
Extended analysis of the phase III KEYNOTE-426 study upholds pembrolizumab plus axitinib as a preferred front-line regimen over sunitinib in patients with advanced sporadic renal cell carcinoma.1 These updated results were presented during the ASCO20 Virtual Scientific Program by Elizabeth R....
Salvage Nivolumab Plus Ipilimumab After Prior Immune Checkpoint Inhibitor Therapy in Patients With Metastatic Renal Cell Carcinoma
In a study reported in the Journal of Clinical Oncology,1 Anita Gul, MD, of the Cleveland Clinic Taussig Cancer Institute, and colleagues found that salvage therapy with nivolumab/ipilimumab was capable of producing a response after prior PD-1 pathway inhibitor therapy in some patients with...
First-Line Combination Treatments Improve Outcomes vs Sunitinib in Advanced Renal Cell Carcinoma
Two recently reported phase III trials have shown the benefits of combination therapy vs sunitinib in the first-line treatment of advanced renal cell carcinoma. As reported in The New England Journal of Medicine by Brian I. Rini, MD, of Cleveland Clinic Taussig Cancer Institute, and colleagues, the ...
Novel Indications and New Drugs for the Treament of Patients With Renal Cell Carcinoma
Pembrolizumab Plus Axitinib: On April 19, 2019, pembrolizumab (Keytruda) was approved for use in combination with the small-molecule tyrosine kinase inhibitor axitinib (Inlyta) for the first-line treatment of patients with advanced renal cell carcinoma. Approval was based on findings in the phase...
Immune Checkpoint Inhibitor Rechallenge in Metastatic Renal Cell Carcinoma
In a study presented during the ASCO20 Virtual Scientific Program1 and published as a brief report in JAMA Oncology,2 Praful Ravi, MB, BChir, of the Dana-Farber Cancer Institute, Boston, and colleagues found that rechallenge with immune checkpoint inhibitor therapy was capable of producing...
Genitourinary Oncology Highlights 2019–2020 Almanac
Over the past year, we have seen significant advances in the treatment of prostate, kidney, and urothelial cancers that will benefit patients now and in the future. We have learned about the final results of important clinical trials across multiple genitourinary cancers disease states leading to...
FDA Pipeline: Designations in Kidney and Lung Cancers, Myelodysplastic Syndromes, and More
Over the past few weeks, the U.S. Food and Drug Administration (FDA) has issued designations and accepted applications for novel agents, as well as approved companion diagnostics. We summarize these regulatory movements below. Breakthrough Therapy Designation for MK-6482 in von Hippel-Lindau...
Savolitinib vs Sunitinib in MET-Driven Papillary Renal Cell Carcinoma
Targeting MET alterations with savolitinib appears to be a better strategy than sunitinib for patients with MET-driven papillary renal cell carcinoma, according to results of the open-label, randomized, phase III SAVOIR trial.1 Patients with MET-driven metastatic papillary renal cell carcinoma...
Study Supports Pembrolizumab Plus Axitinib in Previously Untreated Advanced Renal Cell Carcinoma
Extended analysis of the phase III KEYNOTE-426 study upholds pembrolizumab plus axitinib as a preferred front-line regimen over sunitinib in patients with advanced sporadic renal cell carcinoma.1 These updated results were presented at the ASCO20 Virtual Scientific Program by Elizabeth R. Plimack,...
Salvage Nivolumab/Ipilimumab After Prior Immune Checkpoint Inhibitor Therapy in Patients With Metastatic Renal Cell Carcinoma
In a study reported in the Journal of Clinical Oncology, Gul et al found that salvage therapy with nivolumab/ipilimumab was capable of producing a response after prior programmed cell death 1 (PD-1) pathway inhibitor therapy in some patients with metastatic renal cell carcinoma. Study Details The...
FDA Pipeline: Fast Track Designations in Colorectal and Pancreatic Cancers, Lymphoplasmacytic Lymphoma/Waldenström’s Macroglobulinemia
Over the past month, the U.S. Food and Drug Administration has granted Fast Track designation to agents designed to treat colorectal and pancreatic cancers, in addition to lymphoplasmacytic lymphoma/Waldenström’s macroglobulinemia; accepted a new drug application for a treatment for relapsed or...
Percutaneous Cryoablation vs Partial or Radical Nephrectomy for Early-Stage Kidney Cancer
A minimally invasive procedure that destroys cancer cells by freezing them may be an option other than surgery for treating early-stage kidney cancer. The two methods showed similar 10-year survival rates, with cryoablation showing a lower rate of complications, according to a study published by...
Immunogenomic Characteristics of Advanced Clear Cell Kidney Cancer Treated With Checkpoint Inhibitors
By analyzing tumors from patients treated with immunotherapy for advanced kidney cancer in three clinical trials, scientists have identified several features of the tumors that influence their response to immune checkpoint inhibitors. The research was presented during the ASCO20 Virtual Scientific...
Immune Checkpoint Inhibitor Rechallenge in Metastatic Renal Cell Carcinoma
In a study presented at the ASCO20 Virtual Scientific Program (Abstract 5077) and published as a brief report in JAMA Oncology, Ravi et al found that rechallenge with immune checkpoint inhibitor therapy was capable of producing responses in patients with metastatic renal cell carcinoma, including...
Eric Jonasch, MD, on a Novel Therapy for Von Hippel-Lindau Disease–Associated RCC
Eric Jonasch, MD, of The University of Texas MD Anderson Cancer Center, discusses phase II study findings on the oral HIF-2α inhibitor known as MK-6482, which showed efficacy and tolerability in patients with Von Hippel-Lindau (VHL)–associated clear cell renal cell carcinoma as well as responses in other VHL-related lesions (Abstract 5003).
Expert in Clinical Trial Methodology Makes His Mark in Genitourinary Cancer
In 2019, at the ASCO Annual Meeting, Ian Tannock, MD, PhD, DSc, FASCO, was honored with the Allen S. Lichter Visionary Award for his contributions to the fields of genitourinary and breast cancers as well as his efforts to optimize clinical trial design. The title of his lecture was “Clinical...
HIF2A Inhibitor for von Hippel-Lindau Disease–Associated Renal Cell Carcinoma
In an international trial, treatment with MK-6482, a small-molecule inhibitor of hypoxia-inducible factor (HIF)-2a, was well tolerated and resulted in clinical responses for patients with von Hippel-Lindau disease–associated renal cell carcinoma (RCC). The results of the phase II trial were shared ...
Can Pain After Prostatectomy or Nephrectomy Be Managed Without Opioids?
The use of opioids continues to be major issue facing patients with cancer in the United States. Most patients undergoing prostate and kidney removal may be managed effectively without opioids during the postoperative period, according to new data from researchers in Pittsburgh highlighted during a ...
FDA Pipeline: Two Breakthrough Therapy Designations for Fam-Trastuzumab Deruxtecan-nxki, and More
Over the past few weeks, the U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy designations for an antibody-drug conjugate in the treatment of gastric and lung cancers. The Agency has also issued Orphan Drug designations for agents being investigated in chronic myeloid...
Selected Poster Presentations on Cancer Therapeutics and More
Although the live 2020 National Comprehensive Cancer Network (NCCN) Annual Conference was canceled, more than 100 posters scheduled for presentation are now available online, as part of the NCCN 2020 Virtual Annual Conference. The ASCO Post has summarized some of the clinical trial updates we found ...
Brief Highlights on Novel Therapies for Prostate, Bladder, and Kidney Cancers
Attendees gathered at the 2020 Genitourinary Cancers Symposium in San Francisco to hear the latest news about treating patients with cancers of the prostate, bladder, kidneys, and testicles. In addition to the comprehensive coverage of the meeting in The ASCO Post, here are some brief highlights...
Adding Radiotherapy to Immunotherapy in Renal Cell Carcinoma: Studies Find Mixed Results
Despite recent enthusiasm for combining stereotactic body radiation therapy with immunotherapy in renal cell carcinoma, two preliminary studies presented at the 2020 Genitourinary Cancers Symposium suggest that it may not be the best path forward. In one study, the combination of nivolumab plus...
Addition of Vincristine/Irinotecan to Established Chemotherapy Regimen in Patients With Diffuse Anaplastic Wilms Tumor
In a Children’s Oncology Group study (AREN0321) reported in the Journal of Clinical Oncology, Daw et al found that the addition of vincristine/irinotecan to a regimen used in the National Wilms Tumor Study 5 (NWTS-5; vincristine, doxorubicin, cyclophosphamide, carboplatin, and etoposide plus...
Expert Point of View: Navid Hafez, MD, MPH
Ilixadencel is essentially a dendritic cell vaccine without preloaded antigens. In the MERECA study, ilixadencel produced “a great signal,” though this approach is still very experimental, said the study’s invited discussant, Navid Hafez, MD, MPH, of Yale Cancer Center. Dr. Hafez is a member of the ...
Off-the-Shelf Dendritic Vaccine Shows Benefit in Kidney Cancer
Ilixadencel is a cell-based, allogeneic, off-the-shelf product aimed at priming the anticancer immune response when injected intratumorally. The phase II MERECA study evaluated this allogeneic dendritic cell product given with sunitinib in 88 patients with metastatic renal cell carcinoma. The study ...
Expert Point of View: Daniel Geynisman, MD
Formal discussant of this trial of MK-6482, Daniel Geynisman, MD, of Fox Chase Cancer Center, Philadelphia, was enthusiastic about this presentation. “The response rates were fabulous in this group of heavily pretreated patients,” he stated. “A total of 69% had some tumor shrinkage, 24% had an...
MK-6482 Shows Activity Across All Risk Categories in Metastatic Clear Cell Kidney Cancer
A novel approach using a drug called MK-6482 showed activity in a phase I/II study in patients with metastatic clear cell renal cell carcinoma, according to a presentation at the 2020 Genitourinary Cancers Symposium.1 MK-6482 is an oral, first-in-class selective small-molecule inhibitor that...
Pembrolizumab Plus Bevacizumab for Metastatic Renal Cell Carcinoma
In a phase Ib/II trial (BTRC-GU14-003) reported in the Journal of Clinical Oncology, Dudek et al found that the combination of pembrolizumab and bevacizumab showed activity in patients with metastatic clear cell renal cell carcinoma. As stated by the investigators, “Anti-angiogenic treatment has...
Ziad Bakouny, MD, on Sarcomatoid and Rhabdoid RCC: Potential Determinants of Poor Prognosis and Treatment Response
Ziad Bakouny, MD, of Dana-Farber Cancer Institute, discusses two types of renal cell cancer that are associated with poor prognosis. Because recent early data suggest these tumors respond well to immune checkpoint inhibitors, the authors characterized the tumors in an integrative molecular and clinical study (Abstract 715).
Toni K. Choueiri, MD, on COSMIC-313: Cabozantinib in Combination With Nivolumab/Ipilimumab in RCC
Toni K. Choueiri, MD, of Dana-Farber Cancer Institute, describes a currently recruiting phase III study (COSMIC-313) of cabozantinib in combination with nivolumab and ipilimumab vs nivolumab/ipilimumab for patients with previously untreated advanced renal cell carcinoma of intermediate or poor risk (Abstract TPS767).
Nizar M. Tannir, MD, on Nivolumab/Ipilimumab vs Sunitinib in Advanced Kidney Cancer
Nizar M. Tannir, MD, of The University of Texas MD Anderson Cancer Center, discusses overall survival and an independent review of response in CheckMate 214 with 42-month follow-up, using first-line nivolumab plus ipilimumab vs sunitinib in patients with advanced renal cell carcinoma (Abstract 609).
Thomas Powles, MD, PhD, on Adding Radiation to Immunotherapy for RCC: Improving Systemic Control Through Local Therapy
Thomas Powles, MD, PhD, of Queen Mary University of London, summarizes two papers on metastatic renal cell carcinoma for which he was the discussant: nivolumab in combination with stereotactic body radiotherapy in pretreated patients, and combining dual immune checkpoint inhibition with stereotactic radiation (Abstracts 613 & 614).
Toni K. Choueiri, MD, on an HIF2A Inhibitor for Advanced Clear Cell RCC
Toni K. Choueiri, MD, of Dana-Farber Cancer Institute, discusses findings from a phase I/II trial that found MK-6482 was well tolerated and demonstrated activity in heavily pretreated patients with clear cell renal cell carcinoma (Abstract 611).
Ziad Bakouny, MD, on Metastatic Kidney Cancer: Defining the Role of Cytoreductive Nephrectomy
Ziad Bakouny, MD, of Dana-Farber Cancer Institute, discusses the controversial and ill-defined role of cytoreductive nephrectomy in treating patients with metastatic renal cell carcinoma who have received targeted therapies or immune checkpoint inhibitors (Abstract 608).
2020 GU Cancers Symposium: Fear of Recurrence, Patients’ Prognostic Understanding Examined in Genitourinary Cancers
In studies to be presented at the 2020 Genitourinary Cancers Symposium (Abstracts 649 and 665), researchers examined the prevalence of fear of cancer recurrence in patients with renal cell carcinoma and evaluated the prognostic understanding patients with genitourinary cancer possess of their...
2020 GU Cancers Symposium: Oral HIF2A Inhibitor for Advanced Clear Cell Renal Cell Carcinoma
A novel, first-in-class, small molecule, hypoxia-inducible factor 2 alpha (HIF2A) inhibitor showed single-agent activity in heavily pretreated patients with metastatic clear cell renal cell carcinoma. These results from a phase I/II study will be presented by Toni Choueiri, MD, and colleagues at...
ASCO-SITC 2020: Ilixadencel/Sunitinib vs Sunitinib Alone in Synchronous Metastatic Kidney Cancer
In a study presented by Lindskog et al at the 2020 ASCO-SITC Clinical Immuno-Oncology Symposium (Abstract 11), researchers found ilixadencel, a cell-based allogeneic off-the-shelf product, in combination with sunitinib produced a higher objective response rate than sunitinib alone in patients with...
Three Studies Examine Relationship Between B-Cell Enrichment and Response to Immunotherapy
The likelihood of a patient responding to immune checkpoint blockade may depend on B cells in the tumor, located within specialized immune-cell clusters known as tertiary lymphoid structures, according to three studies all recently published in Nature. The studies showed that enrichment of B cells...
TIVO-3: Third- or Fourth-Line Tivozanib vs Sorafenib in Metastatic Renal Cell Carcinoma
As reported in The Lancet Oncology by Brian I. Rini, MD, and colleagues, the phase III TIVO-3 trial has shown a statistically significant increase in progression-free survival with tivozanib vs sorafenib as a third- or fourth-line treatment for advanced renal cell carcinoma. Study Details The...
First-Line Pembrolizumab Plus Axitinib for Advanced Renal Cell Carcinoma
On April 19, 2019, pembrolizumab was approved for use in combination with the small-molecule tyrosine kinase inhibitor axitinib for the first-line treatment of patients with advanced renal cell carcinoma.1,2 Supporting Efficacy Data Approval was based on findings in the open-label phase III...
Immunotherapy Strategies in Renal Cell Carcinoma: Present and Future
Immunotherapy with checkpoint inhibitors is now considered a standard of care for the front-line treatment of advanced renal cell carcinoma. Despite better outcomes with these agents, there is still room for improvement. At the 2019 Chemotherapy Foundation Symposium, Robert J. Motzer, MD, of...
Atezolizumab/Bevacizumab in Metastatic Renal Cell Carcinoma With Variant Histology or Sarcomatoid Features
In a phase II trial reported in the Journal of Clinical Oncology, Toni K. Choueiri, MD, and colleagues found that the combination of atezolizumab and bevacizumab was active in metastatic renal cell carcinoma with variant histology and clear cell renal cell carcinoma with ≥ 20% sarcomatoid...
ESMO Congress 2019: Quick Takes From Key Clinical Trials
The ESMO Congress continues to grow as a pivotal platform for research in clinical oncology. At the ESMO Congress 2019, important findings were showcased in more than 2,200 studies, including 93 late-breaking abstracts. The ASCO Post summarized much of that news in separate articles over several...
Immunotherapy Combinations Redefine Outcomes for Patients With Advanced Renal Cell Carcinoma
The treatment landscape for patients with advanced renal cell carcinoma has changed drastically over the past several years with the introduction of many new therapeutic options for patients. The revolution began with the U.S. Food and Drug Administration (FDA) approval of nivolumab and ipilimumab...