
Robert J. Motzer, MD

Daniel Cho, MD
Immunotherapy with checkpoint inhibitors is now considered a standard of care for the front-line treatment of advanced renal cell carcinoma. Despite better outcomes with these agents, there is still room for improvement. At the 2019 Chemotherapy Foundation Symposium, Robert J. Motzer, MD, of Memorial Sloan Kettering Cancer Center, New York, reviewed major trials that led to the use of three different immunotherapy combinations as front-line therapy for advanced clear cell renal cell carcinoma.1 In addition, Daniel Cho, MD, Director of the Developmental Therapeutics Program at Perlmutter Cancer Center at NYU Langone, New York, emphasized that further advances in treating advanced renal cell carcinoma would depend on deepening responses and extending the duration of responses to immunotherapy.2 Dr. Cho also described the potential of next-generation immunotherapy to achieve these goals.
Immunotherapy Combinations Under Study
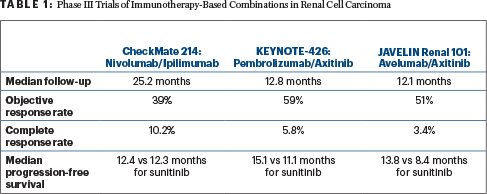
The tyrosine kinase inhibitor sunitinib has been the standard of care in renal cell carcinoma for many years. The phase III randomized trials compared new immunotherapy-based combinations vs sunitinib (Table 1): CheckMate 214 (nivolumab plus ipilimumab vs sunitinib, n = 1,096)3; KEYNOTE-426 (pembrolizumab plus the tyrosine kinase inhibitor axitinib vs sunitinib, n = 861)4; and JAVELIN Renal 101 (avelumab plus axitinib vs sunitinib, n = 886).5 Dr. Motzer told listeners that nivolumab/ipilimumab improved overall survival by 32%; pembrolizumab/axitinib improved overall survival by 47%; and overall survival data are not mature for avelumab/axitinib.
Going forward with new therapies, the quality of response may become the most important endpoint in evaluating immunotherapy regimens.— Daniel Cho, MD
Tweet this quote
When Dr. Motzer drilled down into response data from the three trials, both CheckMate 214 and KEYNOTE-426 showed improved objective response rates in intermediate- and poor-risk patients with the immunotherapy combinations vs sunitinib. However, the response rate in favorable-risk patients was higher for single-agent sunitinib than nivolumab/ipilimumab in CheckMate 214. Dr. Motzer noted that over time, durable complete response rates are higher in favorable-risk patients on nivolumab/ipilimumab vs sunitinib, and survival is similar in both arms.
Based on the results of these three pivotal trials, Dr. Motzer said that nivolumab/ipilimumab or pembrolizumab are both options for front-line therapy for intermediate- and poor-risk patients. “The choice is often individual, based on risks/benefits for each patient. All things aside, I tip toward nivolumab/ipilimumab, because the data are more mature and the quality-of-life data are available,” Dr. Motzer told the audience.
For favorable-risk disease, the National Comprehensive Cancer Network (NCCN) lists pembrolizumab/axitinib or a single-agent tyrosine kinase inhibitor as preferred front-line therapy. “Nivolumab/ipilimumab is also a possibility, providing that the patient and the doctor feel the safety profile is acceptable,” he added.
Novel Approaches
“The robust activity of first-line immunotherapy in patients with renal cell carcinoma has raised the bar for future therapies. To advance the field further, we now need to improve upon response quality with more durable and complete responses. Combination regimens will build on the established efficacy of anti–programmed cell death protein 1 (PD-1) and anti–programmed cell death ligand 1 (PD-L1) antibodies,” Dr. Cho told listeners.2 Current standard immunotherapy for renal cell carcinoma includes nivolumab plus ipilimumab, axitinib plus pembrolizumab, avelumab plus pembrolizumab, and high-dose interleukin-2 (IL-2).
Ongoing phase III trials are comparing the combination of a tyrosine kinase inhibitor plus a checkpoint inhibitor. The CLEAR trial will compare lenvatinib plus everolimus vs lenvatinib plus pembrolizumab vs sunitinib. The COSMIC 313 trial will compare cabozantinib plus ipilimumab/nivolumab vs ipilimumab/nivolumab. “If these trials are positive, new combinations will be added to the list of standard therapies,” Dr. Cho told listeners.
Expectations with current U.S. Food and Drug Administration–approved immunotherapies include high response rates, extended overall survival, extended progression-free survival, and durable responses in most patients. However, complete responses are achieved in few patients. When progression-free survival curves of immunotherapy in renal cell carcinoma are considered, it appears that patients who achieve durable responses are mainly those in complete response.
“Going forward with new therapies, the quality of response may become the most important endpoint in evaluating immunotherapy regimens,” stated Dr. Cho. “Durable and complete responses show the true potential of immunotherapy, which is the ability to cure patients with advanced renal cell carcinoma.”
Promising Strategies
Dr. Cho elaborated on five potential strategies that could improve outcomes: novel immune checkpoint combinations, cytokines, metabolic pathways, vaccines, and cellular therapies. “Novel immune checkpoint combinations allow us to build on the success of checkpoint inhibitors. Combinations can be co-inhibitory and co-stimulatory. There are a mind-numbing number of combination trials of PD-1 antibodies out there,” he continued.
Specifically, combinations being studied in early clinical trials include the following regimens: PD-1/PD-L1 antibodies with LAG-3 antibodies (eg, relatlimab/nivolumab; RGN 3767/cemiplimab, and XmAb 22841/pembrolizumab); PD-1/PD-L1 antibodies with TIM-3 antibodies (MGB453/spartalizumab, RO712166 [bispecific]); and PD-1/PD-L1 antibody with inducible T-cell co-stimulator antibody (GSK3359609 plus pembrolizumab, KY1044/atezolizumab).
“Novel checkpoint agents to watch include NC318, an anti–Siglec 15 monoclonal antibody, and galectin-9,” he said.
Cytokines
“Cytokines regulate the innate and adaptive immune response. In the past, use of cytokines has been limited by the need to give very large doses,” Dr. Cho told the audience. “Based on the proven efficacy of high-dose IL-2 in renal cell carcinoma, researchers are combining cytokines with checkpoint inhibitors.”
Three types of cytokines are in clinical trials in renal cell carcinoma. IL-2 derivates include bempegaldesleukin (NKTR-214) plus nivolumab vs sunitinib in phase III; ALKS 4230 plus pembrolizumab in phase I; and RO6874281 plus atezolizumab in phase I. ALT-803 (IL-15 derivative) plus multiple checkpoint inhibitors are in phase I development. “This is an exciting cytokine that activates natural killer cells,” Dr. Cho explained. M9241 (IL-12 derivative) plus avelumab is in a phase I trial.
Immunotherapy for Kidney Cancer
- Current data suggest that nivolumab/ipilimumab or pembrolizumab/axitinib are options for front-line treatment of advanced renal cell carcinoma in intermediate- and poor-risk patients.
- For favorable-risk patients, single-agent sunitinib is advised, and, with longer follow-up, nivolumab/ipilimumab may prove to be an option for this risk group.
- Novel combination strategies are being explored to deepen and extend responses achieved with immune checkpoint inhibitor–based therapy.
“Hopefully, these approaches [with cytokines] will pay more dividends and deepen response,” stated Dr. Cho.
Another promising area of active investigation focuses on metabolic targets in the tumor microenvironment, based on strong immunomodulatory effects of these metabolites. Several compounds that inhibit these metabolites are being evaluated in combination with checkpoint inhibitors in early clinical trials (CPI-444 plus atezolizumab; INCB001158 plus pembrolizumab).
Vaccines and Cellular Therapies
Vaccines are of interest in combination with checkpoint inhibitors due to their ability to enhance the stimulation of preexisting recognition of tumor by the immune cells. Several are in development, including GEN-009 (personalized vaccine) and RO719847 plus atezolizumab.
Cellular therapeutics under investigation in renal cell carcinoma and other solid tumors include chimeric antigen receptor (CAR) T cells, which are approved for leukemia and lymphoma. They include TRQ15-01 and HERV-E TCR-transduced T cells. “CAR T-cell therapy is farthest away from prime time in renal cell carcinoma compared with the other modalities,” according to Dr. Cho. “Combination regimens building on the efficacy of PD-1/PD-L1 antibodies remain the most attractive strategies.” ■
DISCLOSURE: Dr. Motzer reported financial relationships with Pfizer, Bristol-Myers Squibb, Novartis, Eisai, Exelixis, Genentech/Roche, and Merck. Dr. Cho reported financial relationships with Nektar, Pfizer, PureTech, Torque, and Hoya.
REFERENCES
3. Motzer RJ, Tannir NM, McDermott DF, et al: Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277-1290, 2018.
4. Rini BI, Plimack ER, Stus V, et al: Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1116-1127, 2019.
5. Motzer RJ, Penkov K, Haanen J, et al: Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1103-1115, 2019.

