February 15, 2012 - Supplement (View All Issues)
Special Supplement: Reports from the 34th San Antonio Breast Cancer Symposium and the 53rd American Society of Hematology Annual Meeting
 The ASCO Post presents this special supplement with comprehensive coverage of important data from the 34th Annual San Antonio Breast Cancer Symposium and the 53rd American Society of Hematology Annual Meeting. In addition to news about the individual abstracts, experts offer their perspectives on...
The ASCO Post presents this special supplement with comprehensive coverage of important data from the 34th Annual San Antonio Breast Cancer Symposium and the 53rd American Society of Hematology Annual Meeting. In addition to news about the individual abstracts, experts offer their perspectives on...
Dual HER2 Blockade Substantially Delays Disease Progression
 There is an emerging theme in HER2-positive breast cancer: The greater the pathway inhibition, the better the outcome. The latest evidence comes from the phase III Clinical Evaluation of Pertuzumab and Trastuzumab (CLEOPATRA) trial of 808 patients with previously untreated metastatic disease. The...
There is an emerging theme in HER2-positive breast cancer: The greater the pathway inhibition, the better the outcome. The latest evidence comes from the phase III Clinical Evaluation of Pertuzumab and Trastuzumab (CLEOPATRA) trial of 808 patients with previously untreated metastatic disease. The...
Bevacizumab Progression-free Survival Benefit Upheld in AVEREL Trial
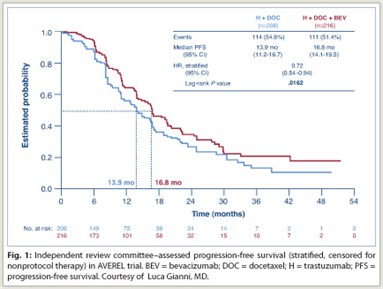 Bevacizumamb (Avastin) added to trastuzumab (Herceptin) and docetaxel as first-line therapy for HER2-positive advanced breast cancer moderately improved progression-free survival in the phase III AVEREL trial, presented at the 2011 San Antonio Breast Cancer Symposium by Luca Gianni, MD, of the San ...
Bevacizumamb (Avastin) added to trastuzumab (Herceptin) and docetaxel as first-line therapy for HER2-positive advanced breast cancer moderately improved progression-free survival in the phase III AVEREL trial, presented at the 2011 San Antonio Breast Cancer Symposium by Luca Gianni, MD, of the San ...
Benefits of Some Bisphosphonates Confirmed in Breast Cancer Outcomes, but Questions Remain
The story of bisphosphonates, and their disease-modifying potential in breast cancer, is still evolving. While some studies presented at the 2011 San Antonio Breast Cancer Symposium failed to show gains, others found benefits. The theme that is emerging is that bisphosphonates may be most...
Can Molecular Profiling Identify ER-positive Patients Destined for Relapse?
Estrogen receptor (ER)-positive breast cancer often recurs years after the initial diagnosis, and understanding the patterns of timing regarding relapse could identify patients needing more aggressive treatment. At the 2011 San Antonio Breast Cancer Symposium, several investigative teams reported...
Everolimus plus Exemestane Significantly Prolongs Remission in BOLERO-2
 Adding an inhibitor of mammalian target of rapamycin (mTOR) to an aromatase inhibitor more than doubled the time to disease progression in patients with advanced, treatment-refractory breast cancer in the phase III BOLERO-2 trial, whose updated results were presented at the San Antonio Breast...
Adding an inhibitor of mammalian target of rapamycin (mTOR) to an aromatase inhibitor more than doubled the time to disease progression in patients with advanced, treatment-refractory breast cancer in the phase III BOLERO-2 trial, whose updated results were presented at the San Antonio Breast...
Post-CHOP Radioimmunotherapy Comparable to Rituximab Given along with CHOP in Previously Untreated Follicular Lymphoma
 In patients with previously untreated follicular lymphoma, similar outcomes were achieved with CHOP-R (cyclophosphamide, doxorubicin, vincristine, prednisone, plus six doses of rituximab [Rituxan]) and CHOP-RIT (CHOP plus one dose of tositumomab/iodine-131 tositumomab [Bexxar]) in a phase III...
In patients with previously untreated follicular lymphoma, similar outcomes were achieved with CHOP-R (cyclophosphamide, doxorubicin, vincristine, prednisone, plus six doses of rituximab [Rituxan]) and CHOP-RIT (CHOP plus one dose of tositumomab/iodine-131 tositumomab [Bexxar]) in a phase III...
BELA Trial Reborn: Bosutinib Produces Improved Results in Chronic Myeloid Leukemia
The initial reports of the BELA trial (Bosutinib Efficacy and safety in chronic myeloid LeukemiA), presented at ASH 2010, were deflating. At 12-month follow-up, bosutinib failed to meet BELA’s primary endpoint. Things have since turned around, however, and results from the 24-month analysis...
Ponatinib Continues to Impress in Patients with Leukemia
 Initial results from the PACE (Ponatinib Ph+ ALL and CML Evaluation) trial added to accumulating evidence that the tyrosine kinase inhibitor ponatinib should soon be considered for front-line treatment of chronic myeloid leukemia (CML).
Initial results from the PACE (Ponatinib Ph+ ALL and CML Evaluation) trial added to accumulating evidence that the tyrosine kinase inhibitor ponatinib should soon be considered for front-line treatment of chronic myeloid leukemia (CML).
“Robust responses were observed in all patient cohorts,” said ...
Three Novel Agents Show Promise in Acute Lymphoblastic Leukemia
 Susan O’Brien, MD, Professor in the Department of Leukemia at The University of Texas MD Anderson Cancer Center, Houston, has a special interest in novel developments in the treatment of acute lymphoblastic leukemia (ALL). At the 2011 ASH Annual Meeting, she discussed her picks of top newsmakers in ...
Susan O’Brien, MD, Professor in the Department of Leukemia at The University of Texas MD Anderson Cancer Center, Houston, has a special interest in novel developments in the treatment of acute lymphoblastic leukemia (ALL). At the 2011 ASH Annual Meeting, she discussed her picks of top newsmakers in ...
Novel Agent Efficacious in Chronic Lymphocytic Leukemia
 A novel inhibitor of B-cell receptor signaling produced high rates of remission and was well tolerated in patients with chronic lymphocytic leukemia (CLL) who were refractory to at least two previous treatments, reported Susan O’Brien, MD, of The University of Texas MD Anderson Cancer Center,...
A novel inhibitor of B-cell receptor signaling produced high rates of remission and was well tolerated in patients with chronic lymphocytic leukemia (CLL) who were refractory to at least two previous treatments, reported Susan O’Brien, MD, of The University of Texas MD Anderson Cancer Center,...
A Second Chance for Gemtuzumab in Acute Myeloid Leukemia
 Gemtuzumab ozogamicin (Mylotarg) may have a second chance for regulatory acceptance, as studies presented at ASH 2011 demonstrated that gemtuzumab can be safely and effectively given by adjusting the dosing and treatment schedule.
Gemtuzumab ozogamicin (Mylotarg) may have a second chance for regulatory acceptance, as studies presented at ASH 2011 demonstrated that gemtuzumab can be safely and effectively given by adjusting the dosing and treatment schedule.
Gemtuzumab ozogamicin was approved for the treatment of acute...
Monoclonal Antibody Promising in Multiple Myeloma
 Elotuzumab, a humanized IgG1 monoclonal antibody targeting human CS1, a cell-surface glycoprotein expressed on 95% of myeloma cells, elicited responses in 82% of relapsed/refractory myeloma patients in a phase II study reported at the ASH Annual Meeting.1 Objective response rates exceeded 90% in...
Elotuzumab, a humanized IgG1 monoclonal antibody targeting human CS1, a cell-surface glycoprotein expressed on 95% of myeloma cells, elicited responses in 82% of relapsed/refractory myeloma patients in a phase II study reported at the ASH Annual Meeting.1 Objective response rates exceeded 90% in...
Next-generation Proteasome Inhibitors Will Improve Outcomes in Bortezomib-refractory Myeloma Patients
The next-generation proteasome inhibitor carfilzomib is expected to gain FDA approval in the near future, offering a treatment option that may be as effective as and less neurotoxic than bortezomib (Velcade). Studies presented at the ASH Annual Meeting upheld benefits of the drug observed in...
New Immunomodulatory Drug Produces Impressive Phase II Results in Multiple Myeloma
 Data on pomalidomide, the novel oral immunomodulatory drug for multiple myeloma, was a major highlight of the 2011 ASH Annual Meeting, according to Kenneth D. Anderson, MD, of Dana-Farber Cancer Institute, Boston, who called the drug “very, very exciting.”
Data on pomalidomide, the novel oral immunomodulatory drug for multiple myeloma, was a major highlight of the 2011 ASH Annual Meeting, according to Kenneth D. Anderson, MD, of Dana-Farber Cancer Institute, Boston, who called the drug “very, very exciting.”
Paul Richardson, MD, also of Dana-Farber...
