Nicoletta Colombo, MD, on Cervical Cancer: KEYNOTE-826 Trial of Pembrolizumab Plus Chemotherapy
ESMO Congress 2021
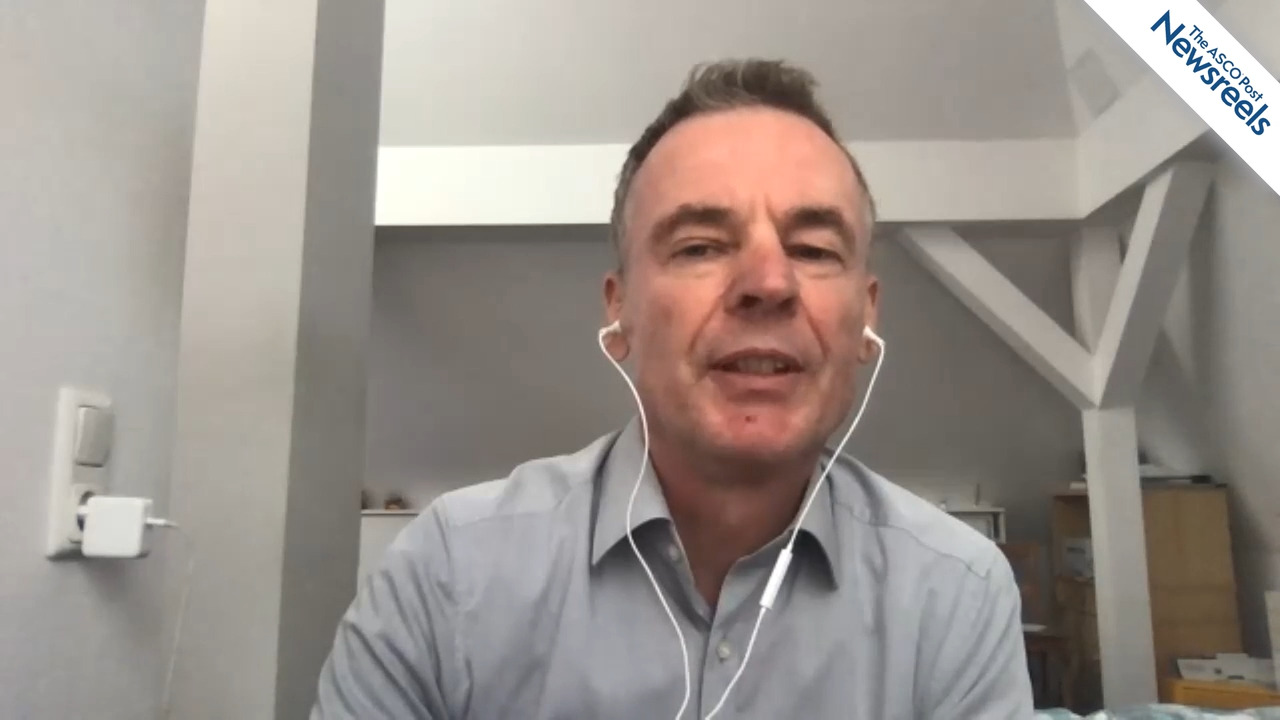
Nicoletta Colombo, MD, of the Istituto Europeo Oncologico, discusses phase III results that showed improvements in progression-free and overall survival with a combination of pembrolizumab plus chemotherapy, compared with placebo and chemotherapy, for patients with persistent, recurrent, or metastatic cervical cancer. These benefits were seen regardless of PD-L1 expression and concomitant bevacizumab use, suggesting that pembrolizumab plus chemotherapy, with or without bevacizumab, may be a new standard of care for this population (Abstract LBA2).
Related Videos
The ASCO Post Staff
Dieter Hörsch, MD, of Germany’s Central Clinic in Bad Berka, discusses phase III results from the SPINET trial, the largest prospective study to date of the somatostatin analog lanreotide autogel. The study suggests that this agent may prove to be an appropriate treatment option for patients with somatostatin receptor–positive bronchopulmonary neuroendocrine tumors, especially typical carcinoids (Abstract 1096O).
The ASCO Post Staff
Javier Cortés, MD, PhD, of Barcelona’s IOB Institute of Oncology, discusses phase III data from the DESTINY-Breast03 study, which support trastuzumab deruxtecan becoming the standard of care for second-line treatment of women with HER2-positive metastatic breast cancer (Abstract LBA1).
The ASCO Post Staff
Hope S. Rugo, MD, of the University of California, San Francisco, discusses phase III results from the KEYNOTE-355 study of pembrolizumab plus chemotherapy, which improved overall survival vs chemotherapy alone in patients with previously untreated locally recurrent, inoperable, or metastatic triple-negative breast cancer whose tumors expressed PD-L1 (Abstract LBA16).
The ASCO Post Staff
Susana N. Banerjee, MBBS, PhD, of The Royal Marsden NHS Foundation Trust, discusses phase I results that have generated interest in the combination of the RAF/MEK inhibitor VS-6766 and the FAK inhibitor defactinib for patients with recurrent low-grade serous ovarian cancer, a disease that typically has limited response to conventional chemotherapy and hormonal therapy. The data support ongoing investigation (Abstract 725MO).
The ASCO Post Staff
Susana N. Banerjee, MBBS, PhD, of The Royal Marsden NHS Foundation Trust, discusses phase II results of the EORTC-1508 trial, the first study to combine an anti–PD-L1 antibody, atezolizumab, with bevacizumab and the COX1/2 inhibitor acetylsalicylic acid as treatment for patients with ovarian, fallopian tube, or primary peritoneal adenocarcinoma (Abstract LBA32).