All-Cause Mortality Risk With Adjuvant vs Early Salvage Radiotherapy After Radical Prostatectomy
In a retrospective cohort study reported in the Journal of Clinical Oncology, Tilki et al found that adjuvant radiotherapy was associated with reduced risk for all-cause mortality vs early salvage radiotherapy among men at high risk for disease recurrence following radical prostatectomy. As stated...
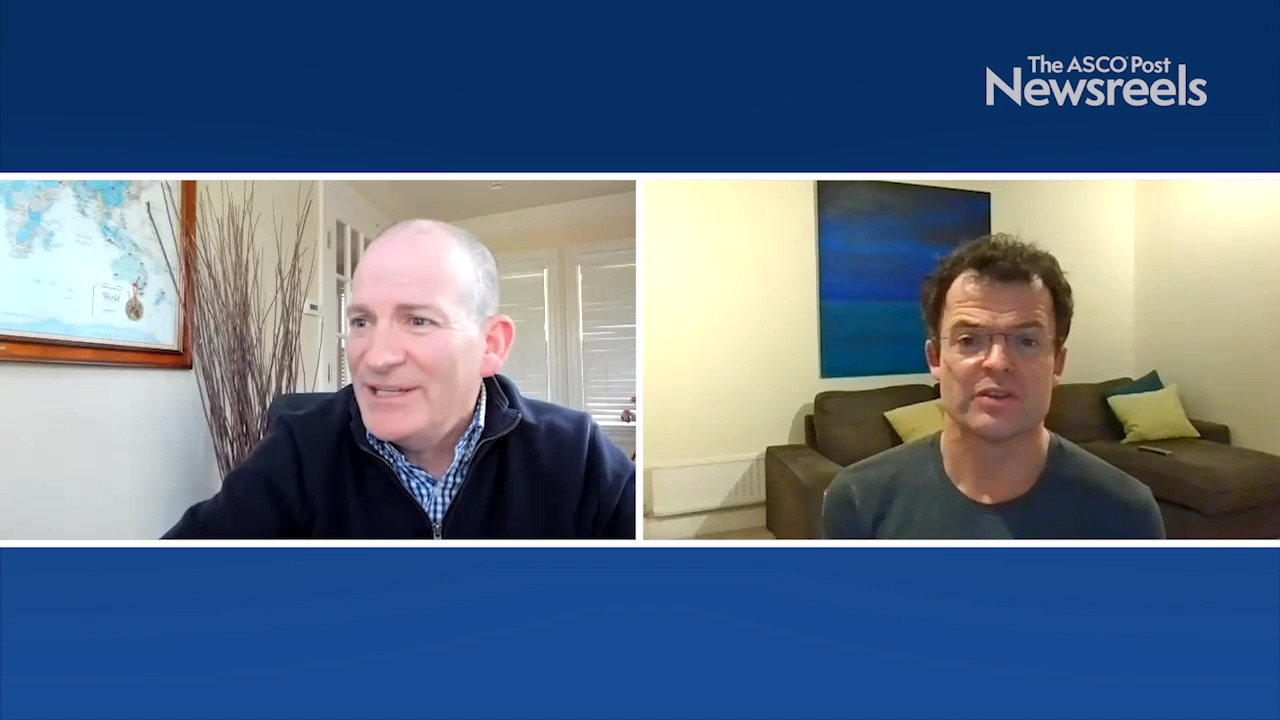
Karim Fizazi, MD, PhD, on Prostate Cancer: Abiraterone Acetate, Prednisone, and Radiotherapy in Metastatic Disease
Karim Fizazi, MD, PhD, of Institut Gustave Roussy, discusses first results from the phase III PEACE1 trial, which showed that abiraterone plus androgen-deprivation therapy and docetaxel improves radiographic progression-free survival in men with de novo metastatic prostate cancer (Abstract 5000).
Novel PSMA-Targeted Radiotherapy Improves Radiographic Progression-Free and Overall Survival in Metastatic Prostate Cancer
Lutetium-177–PSMA-617 (Lu-177–PSMA-617)—an investigational radioligand therapy—significantly improved radiographic progression-free survival and overall survival when added to standard of care compared with standard of care alone for men with metastatic castration-resistant prostate cancer whose...
Michael J. Morris, MD, on Prostate Cancer: LuPSMA in the Metastatic Setting
Michael J. Morris, MD, of Memorial Sloan Kettering Cancer Center, discusses phase III results of the VISION study, which showed that lutetium-177–PSMA-617 (LuPSMA), a targeted radioligand therapy, plus standard-of-care treatment improves radiographic progression-free survival and extends overall survival compared with standard of care alone in men with metastatic castration-resistant prostate cancer (Abstract LBA4).
FDA Approves Piflufolastat F-18 Injection, a PSMA PET Imaging Agent, for the Detection of Metastatic or Recurrent Prostate Cancer
On May 27, the U.S. Food and Drug Administration (FDA) approved piflufolastat F-18 injection (Pylarify), an F-18–labeled prostate-specific membrane antigen (PSMA)-targeted positron-emission tomography (PET) imaging agent, to identify suspected metastasis or recurrence of prostate cancer. This is...
Cancer Has Taught Me to Live With Purpose
I have had two life-threatening cancers over the past 3 decades and can say without equivocation that there is never a good time to get cancer. My first cancer diagnosis happened in 1992, just weeks after I had accepted the position of Chief Executive Officer of Hughes Electronics. The job meant a...
Fluciclovine F-18 PET/CT vs Conventional Imaging Alone in Guiding Postsurgery Salvage Radiotherapy for Prostate Cancer
In a single-institution phase II/III trial (EMPIRE-1) reported in The Lancet, Ashesh B. Jani, MD, and colleagues found that use of fluciclovine F-18 positron-emission tomography/computed tomography (PET/CT) in addition to conventional imaging to guide postprostatectomy salvage radiotherapy was...
2021 Genitourinary Cancers Symposium: Advancements in the Care of Older Adults With Prostate Cancer
The 2021 Genitourinary (GU) Cancers Symposium was held in a virtual format on February 11–13 and featured the latest developments in the understanding and treatment of genitourinary cancers. The impact of prostate cancer therapies on outcomes in older adults continues to be a growing area of...
Increased PSA Screening Linked to Lower Risk of Advanced Prostate Cancer at Diagnosis and Death From the Disease in Younger Black Patients
Younger Black men undergoing frequent prostate-specific antigen (PSA) screening appear to have both a lower risk of metastasis at the time of prostate cancer diagnosis and of fatal disease, according to data from an observational study by Qiao et al presented at a presscast in advance of the 2021...
Apalutamide in Metastatic Castration-Sensitive Prostate Cancer: Final Overall Survival Analysis of the TITAN Trial
As reported in the Journal of Clinical Oncology by Kim N. Chi, MD, and colleagues, the final overall survival analysis of the phase III TITAN trial showed significant benefit with apalutamide plus androgen-deprivation therapy vs placebo plus androgen-deprivation therapy in patients with metastatic...
Prospective Study Explores Prostate Cancer and Treatment Outcomes By Race
A study designed to enroll an equal number of Black and White men with advanced prostate cancer confirmed key findings that have been evident in retrospective analyses and suggest potential new avenues for treating Black patients who disproportionately die of the disease. Researchers at Duke Cancer ...
Variations in Testing and Treatment Across Medical Specialties for Men Initiating Active Surveillance for Prostate Cancer
In a study reported in JCO Oncology Practice, Lai et al found that although the majority of men who have initiated active surveillance for prostate cancer are followed by urologists, some are managed by physicians in other specialties, and that testing and subsequent treatment patterns vary across...
YouTube Videos on Bladder Cancer: Study Focuses on Quality of Content
Social media platforms are valuable tools for educating patients about serious health topics, but they can also spread false and biased information with potentially harmful results, according to recent research published by Stacy Loeb, MD, MSc, and colleagues in European Urology. Researchers...
Safety of Stereotactic Body Radiotherapy for Patients With Multiple Metastases
In the phase I NRG-BR001 trial reported in JAMA Oncology, Chmura et al found that stereotactic body radiotherapy (SBRT) could be used safely to treat multiple metastases in patients with breast, lung, and prostate cancers. Study Details The trial was conducted within a consortium of North...
Genitourinary Oncology Highlights 2020–2021 Almanac
Landmark changes in the treatment of genitourinary cancers have occurred over the past year, as summarized in this year’s Genitourinary Oncology Almanac from The ASCO Post. Starting with our area of focus, metastatic renal cell carcinoma, the saga continues with two more positive phase III trials...
Effect of Genomic Prostate Score Testing on Choice of Active Surveillance in a Predominantly Black Patient Population
In the ENACT study, reported in the Journal of Clinical Oncology, Murphy et al found that use of the 12-gene Genomic Prostate Score (GPS) in a predominantly Black patient population with relatively low-risk prostate cancer tended to be associated with reduced selection of active...
Estimated Shifts in Cancer Incidence and Death Over Next 2 Decades
In the next 2 decades, rankings of incidence and death across cancer types in the United States will undergo important changes, according to new research published by Lola Rahib, PhD, and colleagues in JAMA Network Open. The study estimates that pancreatic cancer is on course to become the...
Expert Point of View: Richard K. Valicenti, MD, FASTRO
“The absolute risk reduction in metastasis ranges from 2% to 12% by 10 years. Given this heterogeneity, there is a strong rationale for better prognostic markers to personalize treatment of prostate cancer. Nearly all men treated with androgen-deprivation therapy have variable side effects that...
Novel Decision-Making Tool for Androgen-Deprivation Therapy for Patients With Prostate Cancer
According to a retrospective study, the combined clinical and cell-cycle risk (CCR) score may be able to accurately predict which patients with intermediate- and high-risk prostate cancer will have little additional benefit from androgen-deprivation therapy added to dose-escalated radiotherapy and...
Mutations in CTCs May Predict Clinical Outcomes in Patients With Metastatic Castration-Resistant Prostate Cancer
Various genetic alterations in circulating tumor cells (CTCs) were associated with clinical outcomes and resistance to hormone therapy in patients with metastatic castration-resistant prostate cancer, according to research published by Gupta et al in Molecular Cancer Research. Although only a...
Does Consumption of Ultra-Processed Food and Drink Increase Colorectal Cancer Risk?
Consumption of ultra-processed food and drink could increase the risk of developing colorectal cancer. This was the conclusion of a large study published by Romaguera et al in Clinical Nutrition based on questionnaires about food behaviors completed by around 8,000 people in Spain. The study, the...
Adding Apalutamide to Abiraterone/Prednisone Extends Radiographic Progression-Free but Not Overall Survival
The phase III ACIS trial met its primary endpoint at 6 months showing that apalutamide plus abiraterone acetate/prednisone (AAP) extended radiographic progression-free survival vs abiraterone acetate/prednisone alone in patients with chemotherapy-naive metastatic castration-resistant prostate...
Comparing Radiotherapy Regimens for Biochemically Recurrent Prostate Cancer After Radical Prostatectomy
A dose-intensified approach to salvage radiotherapy failed to show superiority to a conventional-dose strategy in patients with biochemically recurrent prostate cancer who had undergone radical prostatectomy, according to the phase III SAKK 09/10 trial presented at the 2021 Genitourinary Cancers...
LuPSMA Outperforms Cabazitaxel for Metastatic Castration-Resistant Prostate Cancer in Phase II Trial
Following disease progression on docetaxel, prostate-specific membrane antigen (PSMA)-targeted radionuclide therapy reduced the risk of disease progression or death by 37% vs cabazitaxel in men with metastatic castration-resistant prostate cancer in the TheraP phase II trial reported at the 2021...
Patient Refusal of Provider-Recommended Locoregional Treatment for Prostate Cancer: Sociodemographic Factors
In a study reported in JCO Oncology Practice, Dee et al found that the rate of refusal of provider-recommended locoregional treatment for localized prostate adenocarcinoma has increased over time, with Black and Asian men with intermediate- or higher-risk disease being more likely to refuse such...
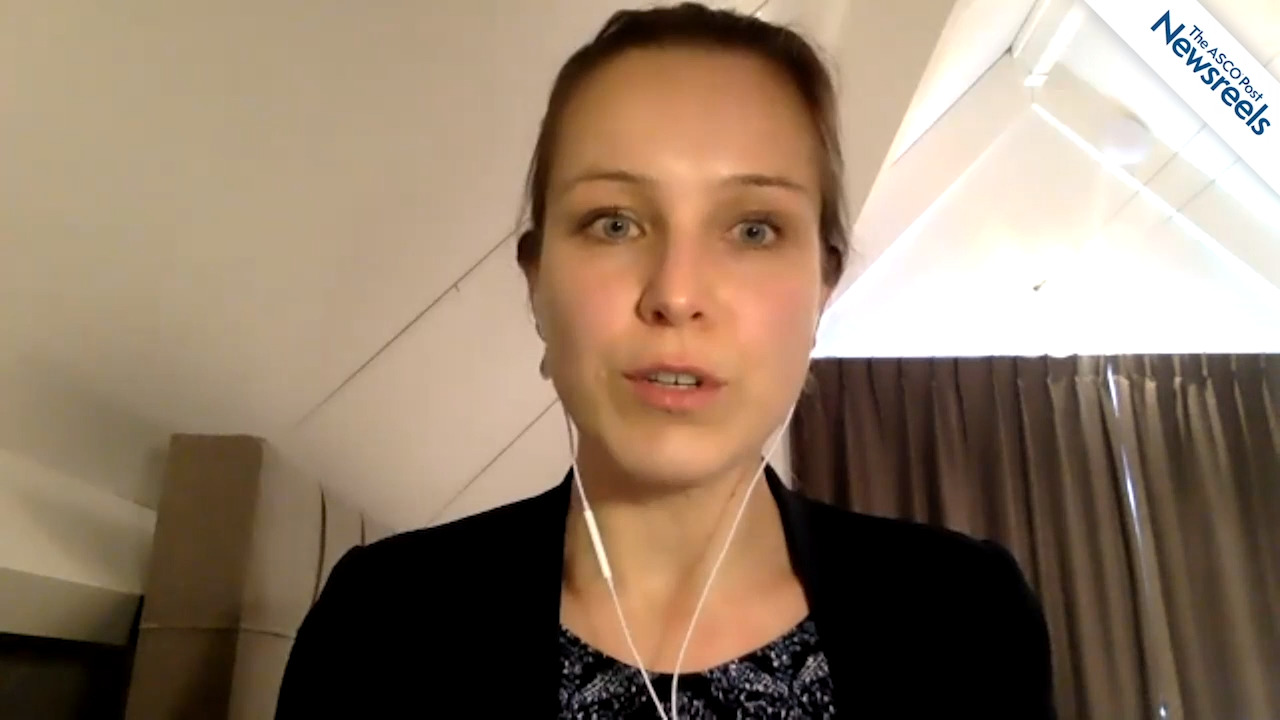
Sandy Srinivas, MD, on Managing Metastatic Castration-Resistant Prostate Cancer
Sandy Srinivas, MD, of Stanford Cancer Institute, discusses the increasing number of ways to deliver life-prolonging therapy to patients with advanced prostate cancer, including more accurate imaging techniques; PET tracers to help better detect, diagnose, and treat disease; PARP inhibitors for BRCA and other mutations; and new sequencing of drugs.
Can Treatment for Prostate Cancer Affect Smell and Taste?
One in six men being treated for advanced prostate cancer reported experiencing a reduced sense of smell and taste, according to a study published by Alonzi et al in the journal Supportive Care in Cancer. The study authors noted that a reduced sense of smell and taste among some patients with...
Meta-analysis of Intermediate Clinical Endpoints as Surrogates for Overall Survival in Localized Prostate Cancer
In a meta-analysis reported in The Lancet Oncology, Gharzai et al found that commonly used intermediate clinical endpoints in clinical trials in localized prostate cancer do not correlate well with overall survival, apart from metastasis-free survival—supporting the establishment of metastasis-free ...
ASCO Guideline Update Offers Four Standards of Care for Noncastrate Advanced, Recurrent, or Metastatic Prostate Cancer
A recent ASCO guideline update, prompted by data from several phase III randomized controlled trials, summarizes the evidence supporting the best initial treatment options for the management of noncastrate advanced, recurrent, or metastatic prostate cancer. The hope is that the guideline will help...
Cardiovascular Events With Transdermal Estradiol vs LHRH Agonists for Androgen Suppression in Advanced Prostate Cancer
In an analysis from the UK phase II/III PATCH trial reported in The Lancet, Langley et al found no differences in cardiovascular event rates over long-term follow-up with the use of a transdermal estradiol patch vs luteinizing hormone-releasing hormone agonists (LHRHa) for androgen suppression in...
Bipolar Androgen Therapy vs Enzalutamide in Asymptomatic Patients With Metastatic Castration-Resistant Prostate Cancer
In the phase II TRANSFORMER trial reported in the Journal of Clinical Oncology, Denmeade et al found no difference in progression-free survival with bipolar androgen therapy—defined as rapid cycling between high and low serum testosterone—vs enzalutamide in men with metastatic castration-resistant...
LuPSMA Leads to Improved PSA Response, Fewer Severe Adverse Events Than Cabazitaxel in Patients With Metastatic Prostate Cancer
In the Australian phase II trial TheraP reported in The Lancet, Michael S. Hofman, FRACP, MBBS, and colleagues found that Lutetium-177–labeled PSMA-617 (LuPSMA) treatment was associated with a higher prostate-specific antigen (PSA) response rate and fewer severe adverse events vs cabazitaxel in...
Bone Metastatic Burden and Survival Outcomes With Prostate Radiotherapy for Newly Diagnosed Metastatic Disease
In an analysis from the STAMPEDE trial reported in JAMA Oncology, Ali et al found that a lower number of bone metastases was associated with improved overall and failure-free survival in patients receiving prostate radiotherapy for newly diagnosed M1 metastatic prostate cancer. Study Details The...
MRI-Targeted Biopsy vs Systematic Transrectal Ultrasonography Biopsy for Detection of Disease in Men at Risk for Prostate Cancer
In a Canadian phase III trial reported in JAMA Oncology, Klotz et al found that multiparametric magnetic resonance imaging (MRI) with targeted biopsy was noninferior to systematic 12-core transrectal ultrasonography biopsy in detecting International Society of Urological Pathology grade group 2...
Felix Y. Feng, MD, on Prostate Cancer: Choosing Patients Who May Benefit From Apalutamide
Felix Y. Feng, MD, of the University of California, San Francisco, discusses study findings showing that molecular determinants may help clinicians select patients with nonmetastatic castration-resistant prostate cancer who may derive the most benefit from apalutamide and other androgen-signaling inhibitors (Abstract 8).
Neeraj Agarwal, MD, on Prostate Cancer: Apalutamide vs Placebo in Treatment of Metastatic Disease
Neeraj Agarwal, MD, of Huntsman Cancer Institute at the University of Utah, discusses final results of the phase III TITAN study, which showed apalutamide plus androgen-deprivation therapy improved overall survival, reducing the risk of death up to 48%. This combination treatment also delayed castration resistance and maintained health-related quality of life for patients with metastatic castration-sensitive prostate cancer (Abstract 11).
Christopher Sweeney, MBBS, and Thomas Powles, MD, PhD, on Treating GU Malignancies: Expert Views
A spirited discussion ensued when we asked Christopher Sweeney, MBBS, of Dana-Farber Cancer Institute, and Thomas Powles, MD, PhD, of Cancer Research UK Barts Centre, to compare notes on how they treat bladder, prostate, and kidney cancers.
LuPSMA vs Cabazitaxel for Metastatic Castration-Resistant Prostate Cancer: Phase II TheraP Trial
Following disease progression on docetaxel, prostate-specific membrane antigen (PSMA)-targeted radionuclide therapy reduced the risk of disease progression or death by 37% vs cabazitaxel in men with metastatic castration-resistant prostate cancer in the phase II TheraP trial reported by Michael S....
Dose-Intensified Radiotherapy Not Superior to Conventional Regimen for Biochemically Recurrent Prostate Cancer After Radical Prostatectomy
A dose-intensified approach to salvage radiotherapy failed to show superiority to a conventional dose strategy in patients with biochemically recurrent prostate cancer who had undergone radical prostatectomy, according to a study presented by Pirus Ghadjar, MD, and colleagues at the 2021...
Update From ACIS Trial of Apalutamide Plus Abiraterone/Prednisone in Metastatic Prostate Cancer
The phase III ACIS trial met its primary endpoint at 6 months, showing that apalutamide plus abiraterone acetate/prednisone extended radiographic progression-free survival vs abiraterone acetate/prednisone alone in patients with chemotherapy-naive metastatic castration-resistant prostate cancer. An ...
Christopher Sweeney, MBBS, on Prostate Cancer: Analyzing Biomarkers, Ipatasertib Plus Abiraterone
Christopher Sweeney, MBBS, of Dana-Farber Cancer Institute, discusses phase III findings from the IPATential150 trial, which showed the effectiveness of ipatasertib plus abiraterone as first-line treatment in patients with metastatic castration-resistant prostate cancer vs placebo plus abiraterone. Analyses of biomarkers linked to the PI3K/AKT pathway, a subtype with a poor prognosis, further support this therapeutic option (Abstract 13).
I Credit Cancer Research With Saving My Life
In December 2015, I thought I was through with cancer. I was diagnosed with prostate cancer in 2011 after a routine blood test showed that my prostate-specific antigen (PSA) level was high. I underwent prostatectomy, and although it was clear the cancer had breached the capsule of the prostate, for ...
Linda G.W. Kerkmeijer, MD, PhD, on Prostate Cancer: Adding a Focal Boost to External-Beam Radiotherapy
Linda G.W. Kerkmeijer, MD, PhD, of the University Medical Center Utrecht and Radboud University Medical Center, discusses results from the phase III FLAME trial, which explored the question of whether biochemical disease–free survival can be improved by adding a focal boost to the intraprostatic lesion in whole-gland external-beam radiotherapy for patients with intermediate- and high-risk prostate cancers (Abstract 126).
Increase in Metastatic Prostate Cancer Diagnoses in the United States After Reduction in PSA Screening
Reduced levels of screening for prostate cancer using prostate-specific antigen (PSA) testing correspond with recent increases in the diagnosis of metastatic disease in the United States, according to a study that will be presented by Vidit Sharma, MD, and colleagues at the 2021 Genitourinary...
Long-Term Quality of Life With Ultrahypofractionated vs Conventionally Fractionated Radiotherapy for Prostate Cancer
As reported in The Lancet Oncology by Fransson et al, analysis of patient-reported quality of life in the Scandinavian phase III HYPO-RT-PC trial showed no significant differences at up to 6 years of follow-up between patients receiving ultrahypofractionated radiotherapy vs conventionally...
Addition of Intraprostatic Focal Boost to External-Beam Radiotherapy in Localized Prostate Cancer
As reported in the Journal of Clinical Oncology by Kerkmeijer et al, the Dutch/Belgian phase III FLAME trial has shown that the addition of a focal boost to the intraprostatic lesion in patients receiving external-beam radiotherapy (EBRT) improved biochemical disease–free survival—without worsening ...
Shorter Radiation Course for Men With Advanced Prostate Cancer: Safety and Efficacy
A study led by researchers from the University of California, Los Angeles (UCLA) Jonsson Comprehensive Cancer Center found that shortening a traditional 45-day course of radiation to a 5-day course delivered in larger doses was safe and as effective as conventional radiation for men with high-risk...
Relugolix for Advanced Prostate Cancer
On December 18, 2020, relugolix was approved for the treatment of adult patients with advanced prostate cancer. Relugolix is the first oral gonadotropin-releasing hormone (GnRH) receptor antagonist to be approved in this setting.1,2 Supporting Efficacy Data Approval was based on findings in the...
Variation in Active Surveillance for Low-Risk Prostate Cancer in the United States
In a cohort study reported in JAMA Network Open, Washington et al found that the use of active surveillance or watchful waiting for patients with low-risk prostate cancer in the United States varied by region, but not according to factors such as Black race or county-level socioeconomic status. As...
FDA Pipeline: Two Reviews in NSCLC, Plus Prescribing Information Update for Darolutamide
Recently, the U.S. Food and Drug Administration (FDA) granted Priority Review to lorlatinib in ALK-positive non–small cell lung cancer (NSCLC) and Breakthrough Therapy designation to the combination of tiragolumab plus atezolizumab in NSCLC with high PD-L1 expression. The FDA also updated the...