Jenny F. Seligmann, MBChB, PhD, on Colorectal Cancer: Adavosertib Compared With Active Monitoring
ESMO Congress 2021
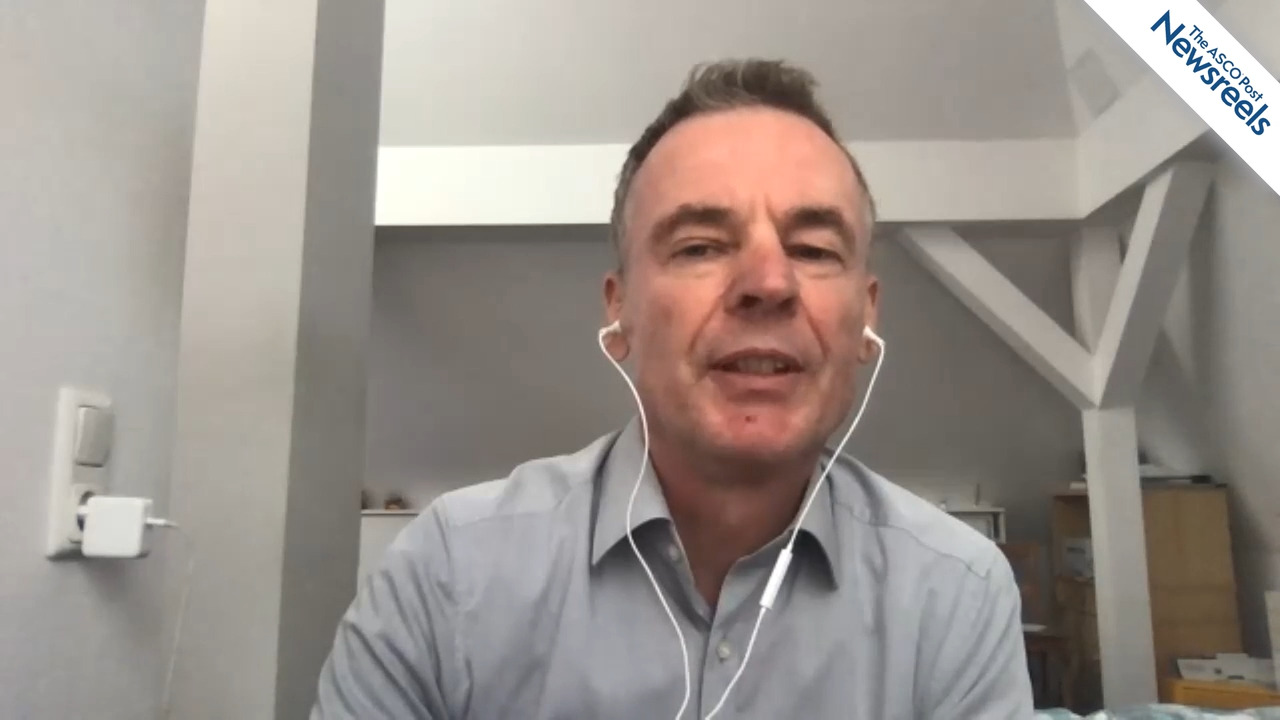
Jenny F. Seligmann, MBChB, PhD, of the University of Leeds, discusses phase II findings that suggest adavosertib improved progression-free survival, compared with active monitoring, by inhibiting the WEE1 kinase in patients with RAS- and TP53-mutant metastatic colorectal cancer. In the trial, adavosertib’s activity tended to be even greater in left-sided tumors (Abstract 382O).
The ASCO Post Staff
Helena M. Earl, MBBS, PhD, of the University of Cambridge, discusses an individual patient data meta-analysis of noninferiority randomized clinical trials to determine whether a duration of less than the standard of 12 months of adjuvant trastuzumab is noninferior for treatment outcomes in patients with HER2-positive early breast cancer (Abstract LBA11).
The ASCO Post Staff
Dieter Hörsch, MD, of Germany’s Central Clinic in Bad Berka, discusses phase III results from the SPINET trial, the largest prospective study to date of the somatostatin analog lanreotide autogel. The study suggests that this agent may prove to be an appropriate treatment option for patients with somatostatin receptor–positive bronchopulmonary neuroendocrine tumors, especially typical carcinoids (Abstract 1096O).
The ASCO Post Staff
Javier Cortés, MD, PhD, of Barcelona’s IOB Institute of Oncology, discusses phase III data from the DESTINY-Breast03 study, which support trastuzumab deruxtecan becoming the standard of care for second-line treatment of women with HER2-positive metastatic breast cancer (Abstract LBA1).
The ASCO Post Staff
Susana N. Banerjee, MBBS, PhD, of The Royal Marsden NHS Foundation Trust, discusses phase I results that have generated interest in the combination of the RAF/MEK inhibitor VS-6766 and the FAK inhibitor defactinib for patients with recurrent low-grade serous ovarian cancer, a disease that typically has limited response to conventional chemotherapy and hormonal therapy. The data support ongoing investigation (Abstract 725MO).
The ASCO Post Staff
Benjamin Besse, MD, PhD, of the Institut Gustave Roussy, discusses final phase III findings from the Atalante-1 trial, which explored the question of whether the OSE2101 vaccine is more beneficial than standard treatment for patients with HLA-A2–positive non–small cell lung cancer after immune checkpoint inhibitors are no longer effective (Abstract LBA47).