FDA Approves Oral Azacitidine as Maintenance Therapy for Adults With AML in First Remission
On September 1, the U.S. Food and Drug Administration (FDA) approved oral azacitidine (Onureg; also known as CC-486) for the continued treatment of adult patients with acute myeloid leukemia (AML) who achieved first complete remission or complete remission with incomplete blood count recovery...
Addition of Nelarabine to Standard Therapy in Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia
As reported in the Journal of Clinical Oncology by Dunsmore et al, the phase III Children’s Oncology Group AALL0434 trial has shown that the addition of nelarabine to standard therapy improved disease-free survival in children and young adults with newly diagnosed T-cell acute lymphoblastic...
Obinutuzumab, Ibrutinib, and Venetoclax in Treatment-Naive and Relapsed or Refractory Chronic Lymphocytic Leukemia
In a single-institution phase II trial reported in the Journal of Clinical Oncology, Rogers et al found that the combination of obinutuzumab, ibrutinib, and venetoclax produced high response rates in both treatment-naive and relapsed or refractory chronic lymphocytic leukemia. Study Details A total ...
Final ASCEND Results Confirm Acalabrutinib as a Standard for Relapsed CLL
The Bruton’s tyrosine kinase (BTK) inhibitors have been one of the most exciting advances in the tre atment of chronic lymphocytic leukemia (CLL) and have led to the development of chemotherapy-free treatments for both treatment-naive as well as relapsed or refractory CLL based on studies where...
Acalabrutinib Improves Progression-Free Survival vs Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in Relapsed or Refractory CLL
As reported in the Journal of Clinical Oncology by Paolo Ghia, MD, PhD, of the Università Vita-Salute San Raffaele, Milan, Italy, and colleagues, the phase III ASCEND trial showed significantly prolonged progression-free survival with acalabrutinib monotherapy vs the investigator’s choice of...
FDA Pipeline: Priority Reviews for Immunotherapy Dose Regimen, Small Cell Lung Cancer; Fast Track Designations in Brain Cancer and Leukemia
Recently, the U.S. Food and Drug Administration (FDA) granted Priority Reviews for a novel dosing regimen for durvalumab as well as for trilaciclib in small cell lung cancer; granted Fast Track designations to treatments for glioblastoma and B-cell acute lymphoblastic leukemia; and issued reports...
Sorafenib Maintenance After Stem Cell Transplant in FLT3-ITD–Positive Acute Myeloid Leukemia
In a Chinese phase III trial reported in The Lancet Oncology, Xuan et al found that sorafenib maintenance after allogeneic hematopoietic stem cell transplantation significantly reduced the risk of relapse vs no maintenance therapy in patients with FLT3–internal tandem duplication (ITD)–positive...
Addition of Venetoclax to Azacitidine in Previously Untreated Patients With AML Ineligible for Induction Therapy
In the phase III VIALE-A trial reported in The New England Journal of Medicine, Courtney D. DiNardo, MD, and colleagues found that venetoclax plus azacitidine significantly improved overall survival vs azacitidine alone in previously untreated patients with acute myeloid leukemia (AML) who were...
FDA Pipeline: Approval for First Liquid Biopsy Next-Generation Sequencing Companion Diagnostic; Designations in Ovarian Cancer, Leukemia, and Lymphoma
Recently, the U.S. Food and Drug Administration (FDA) approved the first liquid biopsy companion diagnostic that also uses next-generation sequencing technology to identify patients with non–small cell lung cancer (NSCLC) and specific types of EGFR mutations. The FDA also granted Fast Track...
ASH Releases New Clinical Practice Guidelines on the Treatment of Older Adults With Newly Diagnosed Acute Myeloid Leukemia
The American Society of Hematology (ASH) has published new guidelines to help older adults with acute myeloid leukemia (AML) and their health-care providers make critical care decisions, including if and how to proceed with cancer treatment and the need for blood transfusions for those in hospice...
Gemtuzumab Ozogamicin in Pediatric Patients With Newly Diagnosed CD33-Positive AML
On June 16, 2020, the indication of gemtuzumab ozogamicin (Mylotarg) for newly diagnosed CD33-positive acute myeloid leukemia (AML) was extended to include pediatric patients aged 1 month and older.1,2 Supporting Efficacy Data Approval was supported by findings from the phase III AAML0531 trial...
Refinement of European LeukemiaNet Classification Recommendations for Younger Adults With AML
Findings from a study published by Eisfeld et al in the journal Leukemia could refine an important set of prognostic and treatment recommendations for younger adult patients with acute myeloid leukemia (AML). The retrospective study evaluated the molecular characteristics and outcomes of 863...
Late Morbidity and Mortality in Survivors of Childhood ALL Receiving Contemporary Risk-Stratified Therapy
In an analysis from the Childhood Cancer Survivor Study reported in the Journal of Clinical Oncology, Dixon et al found that 5-year survivors of childhood acute lymphoblastic leukemia (ALL) diagnosed and treated with risk-stratified therapy in the 1990s had reduced morbidity and health-related late ...
Sorafenib Maintenance After Allogeneic HCT in Patients With FLT3-ITD–Positive AML
In the German-Austrian phase II SORMAIN trial reported in the Journal of Clinical Oncology, Burchert et al found that maintenance treatment with sorafenib vs placebo was associated with significantly prolonged relapse-free survival after allogeneic hematopoietic stem cell transplantation (HCT) in...
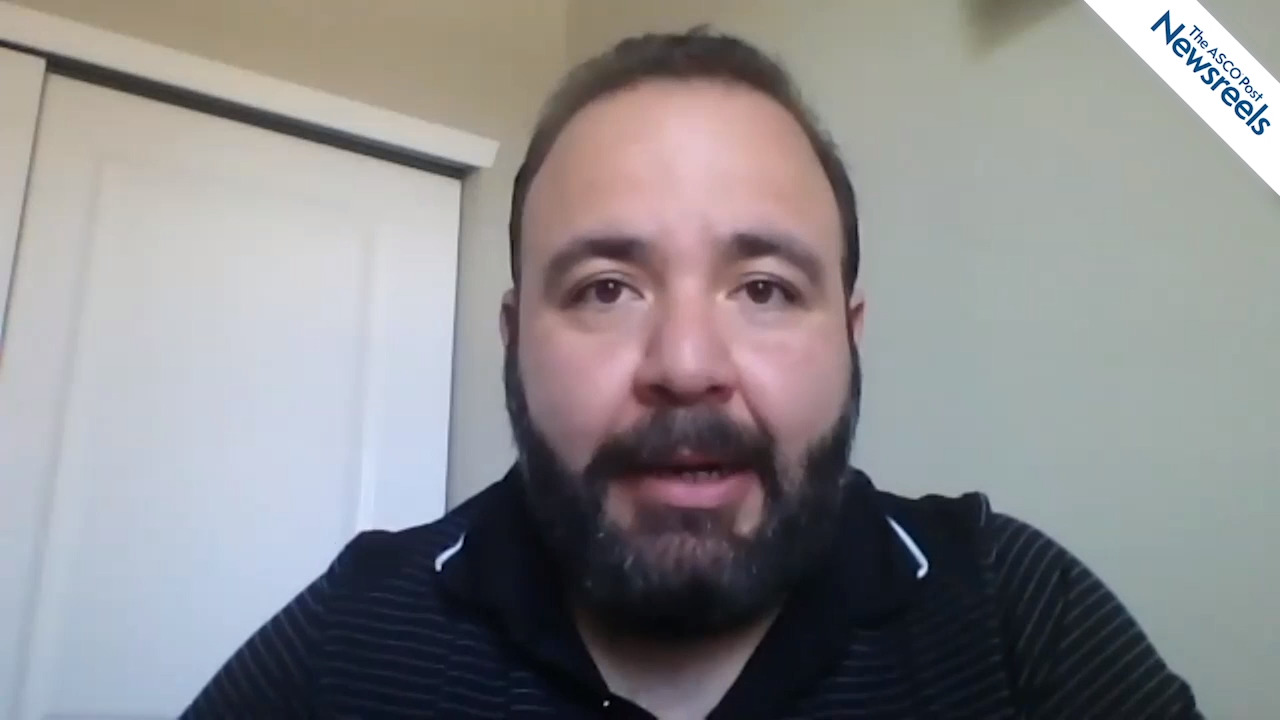
Alfonso Bencomo Álvarez, PhD, on ALL, AML, and CML: Survival for Hispanic Patients Living Near the US/Mexico Border
Alfonso Bencomo Álvarez, PhD, of Texas Tech University Health Sciences Center, discusses his retrospective study of the incidence and survival for patients with hematologic malignancies residing at the United States/Mexico border. The analysis showed that 10-year survival rates for Hispanic patients with ALL, AML, and CML were significantly lower for those who lived in El Paso than for those who lived elsewhere in Texas (Abstract 4343).
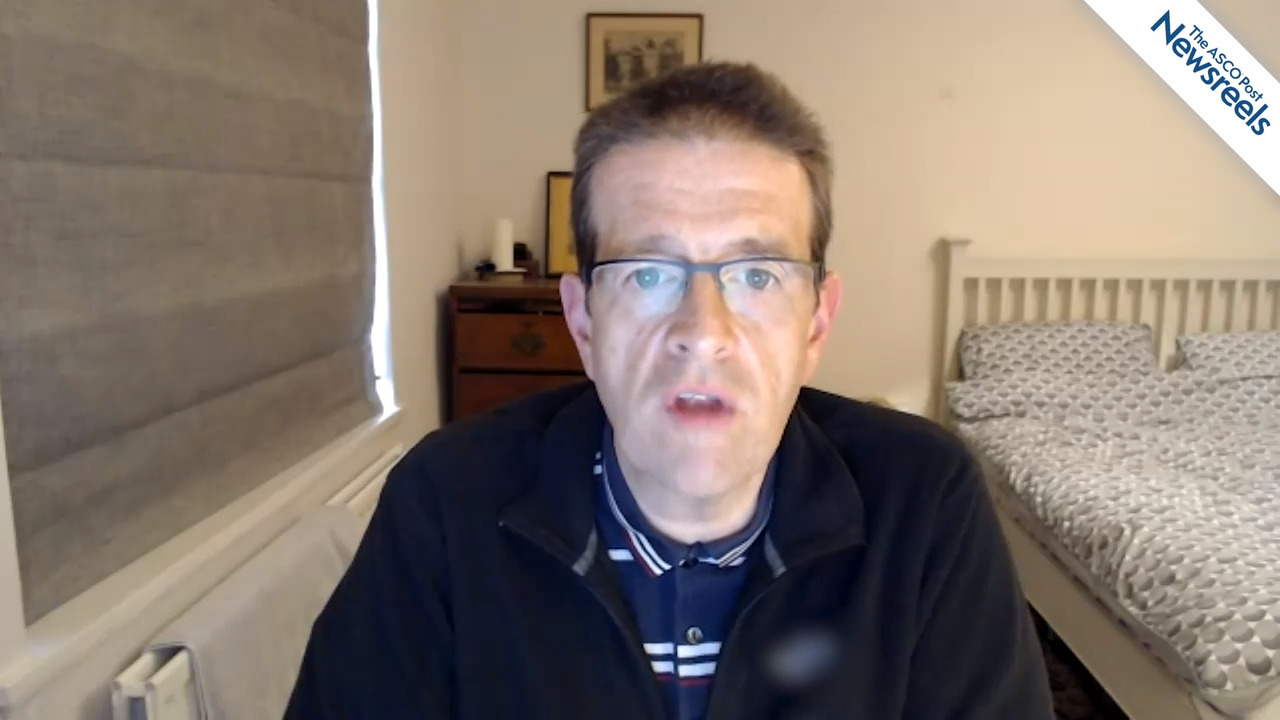
Andrew H. Wei, MBBS, PhD, on AML: Venetoclax Plus Cytarabine in Older Patients
Andrew H. Wei, MBBS, PhD, of The Alfred Hospital, Monash University, discusses phase III data from the VIALE-C trial, which appear to support the use of venetoclax plus low-dose cytarabine as a front-line treatment for older patients with acute myeloid leukemia, as well as for those who cannot tolerate intensive chemotherapy (Abstract S136).
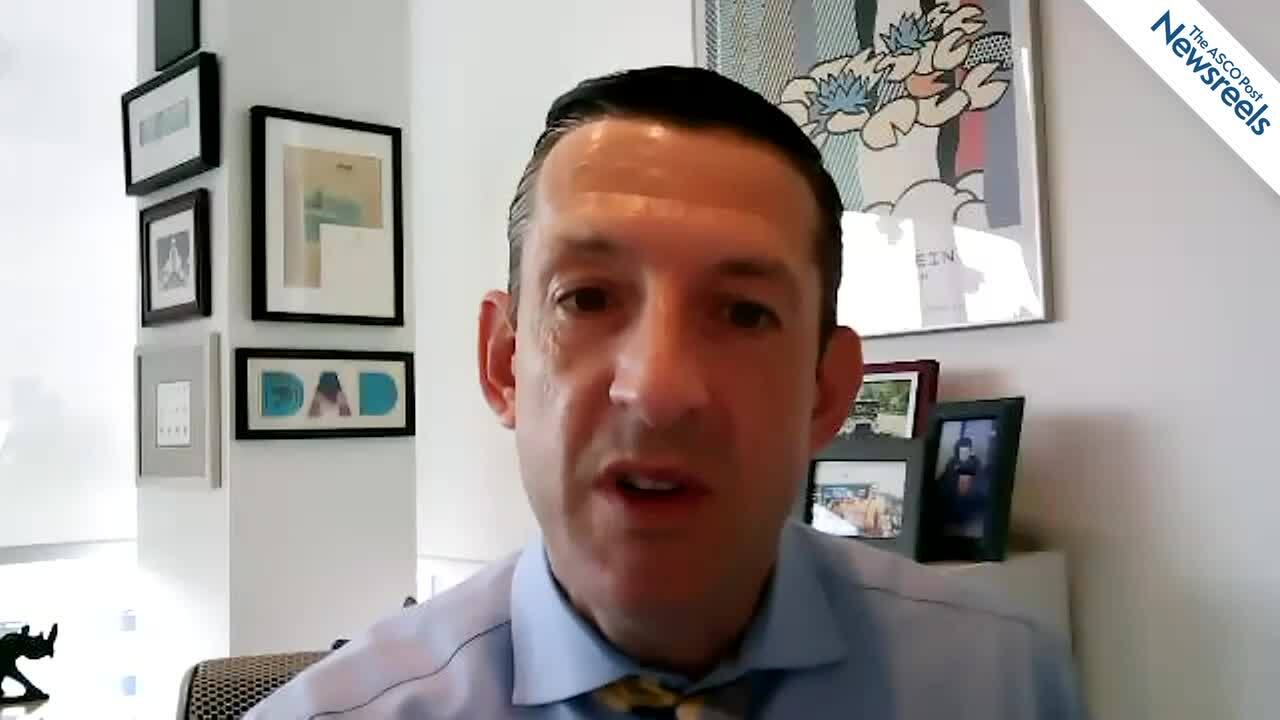
Expert Point of View: Alice Mims, MD
Alice Mims, MD, Associate Professor of Hematology at The Ohio State University Comprehensive Cancer Center–The James, shared her insights on the VIALE-A study. “The results of the VIALE-A study have been highly anticipated and are exciting, given the improvement seen in both overall survival and...
VIALE-A Trial Supports Survival Benefit of Venetoclax Plus Azacitidine in Elderly Patients With AML
In the phase III VIALE-A trial, venetoclax added to azacitidine led to a significant and clinically meaningful improvement in response rates and overall survival, as compared with azacitidine alone, in treatment-naive predominantly elderly patients with acute myeloid leukemia (AML) ineligible for...
Dental Infection Screening Before Induction Chemotherapy for AML
In a single-institution study reported in JCO Oncology Practice, Watson et al found that screening for and treating detected dental infections prior to initiation of induction chemotherapy for patients with newly diagnosed acute myeloid leukemia (AML) were associated with a significant reduction in ...
Arnon P. Kater, MD, PhD, on CLL: Efficacy of Venetoclax in Relapsed or Refractory Disease
Arnon P. Kater, MD, PhD, of the University of Amsterdam, Cancer Center Amsterdam, discusses phase IIIb results from the VENICE I trial, which confirmed that venetoclax monotherapy can achieve deep responses and has a tolerable and manageable safety profile in patients with relapsed or refractory chronic lymphocytic leukemia (Abstract S156).
Jorge E. Cortes, MD, on Chronic Phase CML: Comparing Three Starting Doses of Ponatinib
Jorge E. Cortes, MD, of Georgia Cancer Center, discusses interim results from the OPTIC study, which showed a trend toward dose-dependent efficacy and safety, and may provide a refined understanding of the ponatinib benefit/risk profile and its relation to dose. Mature data from continued follow-up may support an alternate dosing regimen for patients with chronic phase chronic myeloid leukemia (Abstract S172).
John C. Byrd, MD, on CLL/SLL: Acalabrutinib in Treatment-Naive Patients
John C. Byrd, MD, of The Ohio State University Comprehensive Cancer Center, discusses the mature results of a phase II study showing durable remissions and long-term tolerability of acalabrutinib in treatment-naive patients with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma (Abstract S163).
Elias Jabbour, MD, on Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma: Venetoclax and Navitoclax in Relapsed or Refractory Disease
Elias Jabbour, MD, of The University of Texas MD Anderson Cancer Center, discusses study findings that showed venetoclax and navitoclax with chemotherapy is well tolerated, with promising efficacy in heavily pretreated patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Clinical follow-up, correlative biomarker analysis, and expansion cohort enrollment to assess discontinuous dosing are underway (Abstract S116).
Anthony Moorman, PhD, on ALL: Improving Outcomes With a Tyrosine Kinase Inhibitor Plus Chemotherapy
Anthony Moorman, PhD, of Newcastle University, discusses preliminary data showing high-risk patients with acute lymphoblastic leukemia and ABL-class mutations may have improved outcomes when a tyrosine kinase inhibitor is added to chemotherapy (Abstract S117).
Courtney D. DiNardo, MD, on AML: Venetoclax and Azacitidine for Treatment-Naive Patients
Courtney D. DiNardo, MD, of The University of Texas MD Anderson Cancer Center, discusses data from her study of treatment-naive, predominantly older patients with acute myeloid leukemia who are ineligible for intensive therapy. The research shows venetoclax plus azacitidine improved response rates and overall survival compared with azacitidine alone (Abstract LB2601).
Courtney D. DiNardo, MD, on AML: Venetoclax and Azacitidine for Treatment-Naive Patients
Courtney D. DiNardo, MD, of The University of Texas MD Anderson Cancer Center, discusses data from her study of treatment-naive, predominantly older patients with acute myeloid leukemia who are ineligible for intensive therapy. The research shows venetoclax plus azacitidine improved response rates and overall survival compared with azacitidine alone (Abstract LB2601).
Abhishek Maiti, MBBS, on AML: Decitabine and Venetoclax vs Intensive Chemotherapy
Abhishek Maiti, MBBS, of The University of Texas MD Anderson Cancer Center, discusses his analysis showing that 10-day decitabine and venetoclax led to superior outcomes compared with intensive chemotherapy in older patients with acute myeloid leukemia, with benefits most pronounced in people at high risk of treatment-related mortality (Abstract S141).
Acalabrutinib With or Without Obinutuzumab vs Chlorambucil/Obinutuzumab in Previously Untreated CLL
As reported inThe Lancet by Jeff P. Sharman, MD, of Willamette Valley Cancer Institute/US Oncology, Eugene, Oregon, and colleagues, the phase III ELEVATE-TN trial has shown significantly improved progression-free survival with both acalabrutinib/obinutuzumab and acalabrutinib monotherapy vs...
FDA Extends Indication of Gemtuzumab Ozogamicin for Pediatric Patients With CD33-Positive AML
On June 16, the U.S. Food and Drug Administration (FDA) extended the indication of gemtuzumab ozogamicin (Mylotarg) for newly diagnosed CD33-positive acute myeloid leukemia (AML) to include pediatric patients aged 1 month and older. AAML0531 Efficacy and safety in the pediatric population were...
EHA25 Virtual: Roundup of Findings in Leukemia, Lymphoma, and More
Advances in the treatment of hematologic malignancies and some of their associated symptoms were presented during EHA25 Virtual, an ongoing online conference from the European Hematology Association (EHA). Advances in the Treatment of High-Risk CLL: CLL2-GIVe Results In the CLL2-GIVe trial, the...
Acalabrutinib vs Idelalisib/Rituximab or Bendamustine/Rituximab for Relapsed or Refractory CLL: ASCEND Trial
As reported in the Journal of Clinical Oncology by Ghia et al, the phase III ASCEND trial has shown prolonged progression-free survival with acalabrutinib monotherapy vs investigator’s choice of idelalisib plus rituximab or bendamustine plus rituximab in adult patients with relapsed or refractory...
Farhad Ravandi-Kashani, MD, on Acute Myeloid Leukemia: AMG 330 in Patients With Relapsed or Refractory Disease
Farhad Ravandi-Kashani, MD, of The University of Texas MD Anderson Cancer Center, discusses updates from a phase I dose-escalation study of AMG 330, a bispecific T-cell engager molecule. It showed early evidence of an acceptable safety profile, drug tolerability, and antileukemic activity, supporting further dose escalation in patients with acute myeloid leukemia (Abstract 7508).
Mikkael A. Sekeres, MD, on MDS, CMML, or AML: Pevonedistat and Azacitidine
Mikkael A. Sekeres, MD, of the Cleveland Clinic, discusses data from a phase II study of pevonedistat plus azacitidine vs azacitidine alone in patients with higher-risk myelodysplastic syndromes, chronic myelomonocytic leukemia, or low-blast acute myeloid leukemia (Abstract 7506).
Expert Point of View: Yvonne Chen, PhD, and Joseph Alvarnas, MD
Formal discussant Yvonne Chen, PhD, Associate Professor of Microbiology, Immunology, and Molecular Genetics at the University of California, Los Angeles, said the issue of toxicity was important, since high levels of toxicity were observed in this small group of five patients. “All five patients...
Off-the-Shelf CAR T-Cell Therapy Makes Inroads in Acute Lymphoblastic Leukemia
Chimeric antigen receptor (CAR) T-cell therapy known as TruUCAR GC027 may prove to be useful in the treatment of adult patients with relapsed or refractory T-cell acute lymphoblastic leukemia (T-ALL) and potentially other hematologic malignancies. Preliminary results in a small number of patients...
Ibrutinib in Combination With Rituximab in Chronic Lymphocytic Leukemia
On April 21, 2020, ibrutinib was granted an expanded indication for use in combination with rituximab for the initial treatment of adult patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).1,2 The recommended dosage of ibrutinib in CLL/SLL is 420 mg, once daily,...
Liposomal Daunorubicin and Cytarabine Followed by FLAG for Pediatric Relapsed AML
In a phase I/II Children’s Oncology Group study (AAML1421) reported in the Journal of Clinical Oncology, Cooper et al identified the phase II dose of CPX-351, a liposomal preparation of daunorubicin and cytarabine, and found that a regimen consisting of CPX-351 followed by fludarabine, cytarabine,...
Ivosidenib Plus Venetoclax With or Without Azacitidine for IDH1-Mutated AML
Combination therapy with the isocitrate dehydrogenase 1 (IDH1) inhibitor ivosenidib plus the BCL2 inhibitor venetoclax with or without the chemotherapeutic agent azacitidine showed activity in patients with IDH1-mutated acute myeloid leukemia (AML) in a phase Ib/II trial. The results of the...
Treating Patients With Leukemia During the COVID-19 Era at MD Anderson Cancer Center
As part of a series of interviews with cancer experts during the COVID-19 pandemic, The ASCO Post spoke with Hagop Kantarjian, MD, Professor and Chair of the Department of Leukemia at The University of Texas MD Anderson Cancer Center, Houston, about the impact of the pandemic on treatment of...
FDA Pipeline: Two Breakthrough Therapy Designations for Fam-Trastuzumab Deruxtecan-nxki, and More
Over the past few weeks, the U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy designations for an antibody-drug conjugate in the treatment of gastric and lung cancers. The Agency has also issued Orphan Drug designations for agents being investigated in chronic myeloid...
Highlights From ASH 2019 Included New Data in Leukemia, Lymphoma, Myeloma, and Myelodysplastic Syndromes
The 2019 American Society of Hematology (ASH) Annual Meeting & Exposition featured a cornucopia of sessions. It was impossible to attend all the lectures, symposia, oral presentations, poster presentations, and special events because many were concurrent. Below, we have selected some...
Benefit of Increased Dietary Antioxidants During Induction Therapy for Pediatric ALL
In a study reported in the Journal of Clinical Oncology, Ladas et al found that increased dietary intake of antioxidants was associated with reduced risk for bacterial infection and grade ≥ 3 mucositis during induction therapy in pediatric patients with acute lymphoblastic leukemia (ALL). Study...
Laura C. Michaelis, MD: In My Experience Question 5
For community oncologists treating patients with chronic myeloid leukemia or chronic lymphocytic leukemia during the COVID-19 pandemic, what resources and clinical pearls would you suggest? Recorded April 24, 2020.
Laura C. Michaelis, MD: In My Experience Question 4
At this time, would you consider pausing therapy—specifically signaling inhibitors and/or monoclonal antibodies—in patients with chronic lymphocytic leukemia? Recorded April 24, 2020.
Laura C. Michaelis, MD: In My Experience Question 2
Are you treating patients with advanced chronic myeloid leukemia (accelerated phase or blast crisis) any differently during the COVID-19 pandemic? Recorded April 24, 2020.
Laura C. Michaelis, MD: In My Experience Question 1
How has the COVID-19 pandemic changed your approach to the selection of tyrosine kinase inhibitors for the treatment of patients with chronic myeloid leukemia? Recorded April 24, 2020.
Dexrazoxane, Cardiac Function, and Treatment Outcomes in Pediatric AML
In a study reported in the Journal of Clinical Oncology, Getz et al found that use of dexrazoxane was associated with preservation of cardiac function in pediatric patients receiving front-line treatment for acute myeloid leukemia (AML) and resulted in no adverse effects on treatment outcomes. As...
FDA Pipeline: Priority Review in AML, Fast Track Designations for Pancreatic Cancer and Neuroendocrine Tumors
Over the past 2 weeks, the U.S. Food and Drug Administration (FDA) granted Priority Review to a treatment for acute myeloid leukemia (AML); Fast Track designations for agents in pancreatic cancer and pancreatic/nonpancreatic neuroendocrine tumors; approvals for companion diagnostic tests;...
High- vs Low-Dose Anti-CD19 CAR T-Cell Therapy in Patients With Relapsed or Refractory CLL
In a study reported in the Journal of Clinical Oncology by Noelle V. Frey, MD, and colleagues, a higher dose of anti-CD19 chimeric antigen receptor (CAR) T cells was associated with a higher rate of complete response in patients with relapsed or refractory chronic lymphocytic leukemia, with...