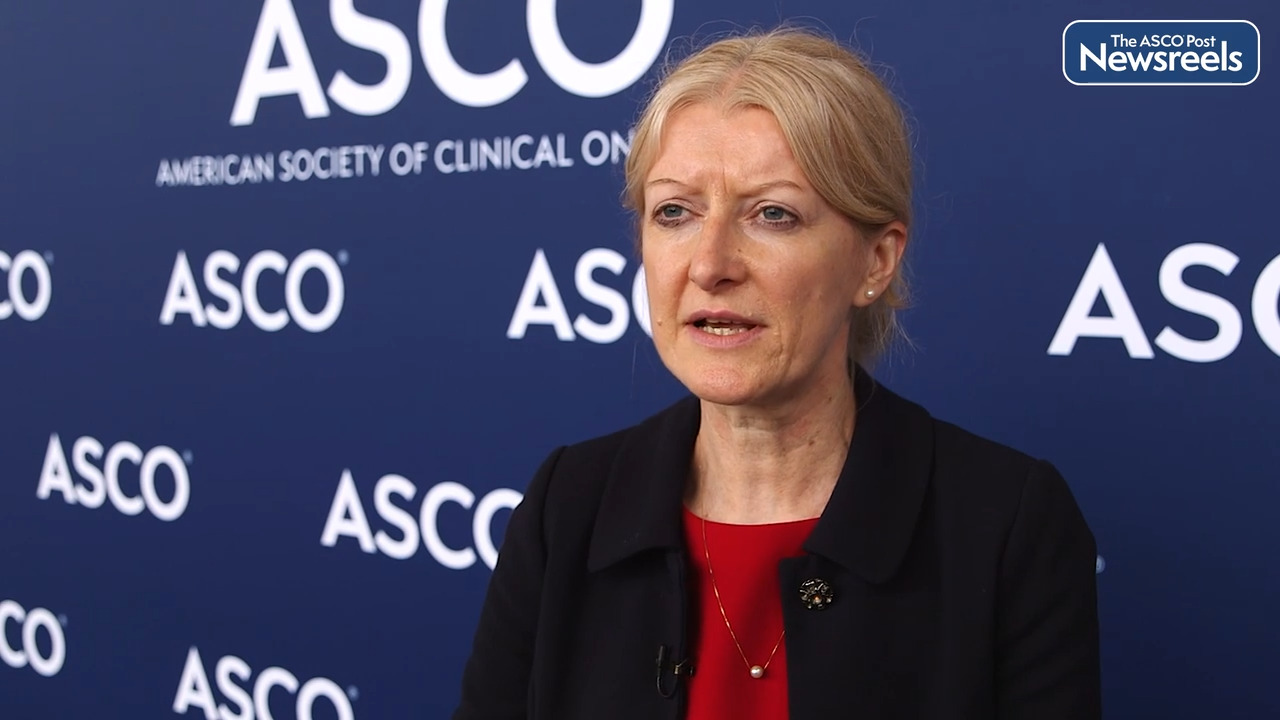Shilpa Gupta, MD, on Urothelial Cancer: Defining Who Is 'Platinum-Ineligible'
2022 ASCO Annual Meeting
Shilpa Gupta, MD, of the Cleveland Clinic Foundation, discusses an updated consensus definition for standard therapy and clinical trial eligibility for patients with metastatic urothelial cancer who are platinum-ineligible, criteria that are proposed to guide treatment recommendations for this population. This may be especially important now that the U.S. Food and Drug Administration has restricted the use of first-line pembrolizumab to those who are considered platinum-ineligible (Abstract 4577).
Transcript
Disclaimer: This video transcript has not been proofread or edited and may contain errors.
Frontline therapy for patients with metastatic urothelial cancer who are Cisplatin ineligible has continued to evolve. And the current standard of care is Gemcitabine and Carboplatin chemotherapy followed by durvalumab maintenance. In 2017, Atezolizumab and Pembrolizumab were approved as single agents for this patient population. But then the label was restricted to patients who are Cisplatin ineligible with high PD-L1 expressing tumors, or those who are not eligible for any platinum. And now Pembrolizumab use is only restricted to patients who are platinum-ineligible. Back in 2019, we presented results from our survey for defining platinum-ineligibility by sending a survey out to around 60 US-based medical oncologists. And we presented a consensus definition at GU ASCO that year. And now with the changing landscape, we updated the survey and used the similar cohort of responders to provide a consensus definition update. So we ask questions like: what equal performance status would physicians use to deem someone platinum-ineligible? What creatinine clearance cutoff would they use? What peripheral neuropathy cutoff, heart failure, cutoff? And in any person with ECOG performance status two, what would be the creatinine clearance cutoff? And based on the majority of responses, we found that most physicians found that creatinine clearance less than 30 milliliters per minute, peripheral neuropathy greater than are equal to grade two, significant heart failure that is NYHA class three or higher, equal performance status greater than our equal to three, and in a patient with equal performance status two, creatinine clearance of less than 30 milliliters per minute. Those were the factors that would make them hesitant to use Carboplatin. So we proposed that if any one of these criteria are met, that patient can be deemed as platinum-ineligible and be a candidate for single agent immunotherapy. Otherwise, we offered Gemcitabine and Carboplatin followed by durvalumab maintenance. Notably age was not a cutoff for these patients based on our survey.
Related Videos
Karim Chamie, MD, of the University of California, Los Angeles, discusses final clinical results on combining the superagonist N-803 with bacillus Calmette-Guérin (BCG) in patients whose carcinoma in situ and high-grade non–muscle-invasive bladder cancers are unresponsive to BCG alone. Of note, cystectomy was avoided in more than 90% of patients with 2 years of follow-up (Abstract 4508).
The ASCO Post Staff
Benoit You, MD, PhD, of Lyon University hospital (HCL, France) and GINECO group (France), discusses findings from the GOG-0218 trial of patients with ovarian cancer, which appears to confirm earlier data on the link between poor tumor chemosensitivity and benefit from concurrent plus maintenance bevacizumab. In Dr. You’s validation study, patients who derived the most progression-free and overall survival benefit from bevacizumab were those with high-risk disease (stage IV or incompletely resected stage III) associated with an unfavorable KELIM score (CA-125 kinetic elimination rate constant, calculable online) (Abstract 5553).
The ASCO Post Staff
Mairéad G. McNamara, PhD, MBBCh, of The Christie NHS Foundation Trust, discusses phase II findings of the NET-02 trial, which explored an unmet need in the second-line treatment of patients with progressive, poorly differentiated extrapulmonary neuroendocrine carcinoma. In the trial, the combination of liposomal irinotecan, fluorouracil, and folinic acid, but not docetaxel, met the primary endpoint of 6-month progression-free survival rate (Abstract 4005).
Lisa A. Carey, MD, of the University of North Carolina Lineberger Comprehensive Cancer Center, and Hope S. Rugo, MD, of the University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center, discuss phase III results from the TROPiCS-02 trial. This study showed that sacituzumab govitecan-hziy was more beneficial than single-agent chemotherapy in terms of progression-free survival in heavily pretreated patients with hormone receptor–positive/HER2-negative and unresectable advanced breast cancer (LBA1001).
The ASCO Post Staff
Courtney D. DiNardo, MD, MSCE, of The University of Texas MD Anderson Cancer Center, and Stéphane de Botton, MD, PhD, of Institut Gustave Roussy, discuss phase III findings from the IDHENTIFY trial, which showed that mutational burden and co-mutational profiles differed between patients with relapsed or refractory acute myeloid leukemia that exhibited IDH2-R140 and IDH2-R172 mutations. Enasidenib improved survival outcomes for patients with IDH2-R172 mutations: median overall survival and 1-year survival rates were approximately double those in the conventional care arm (Abstract 7005).