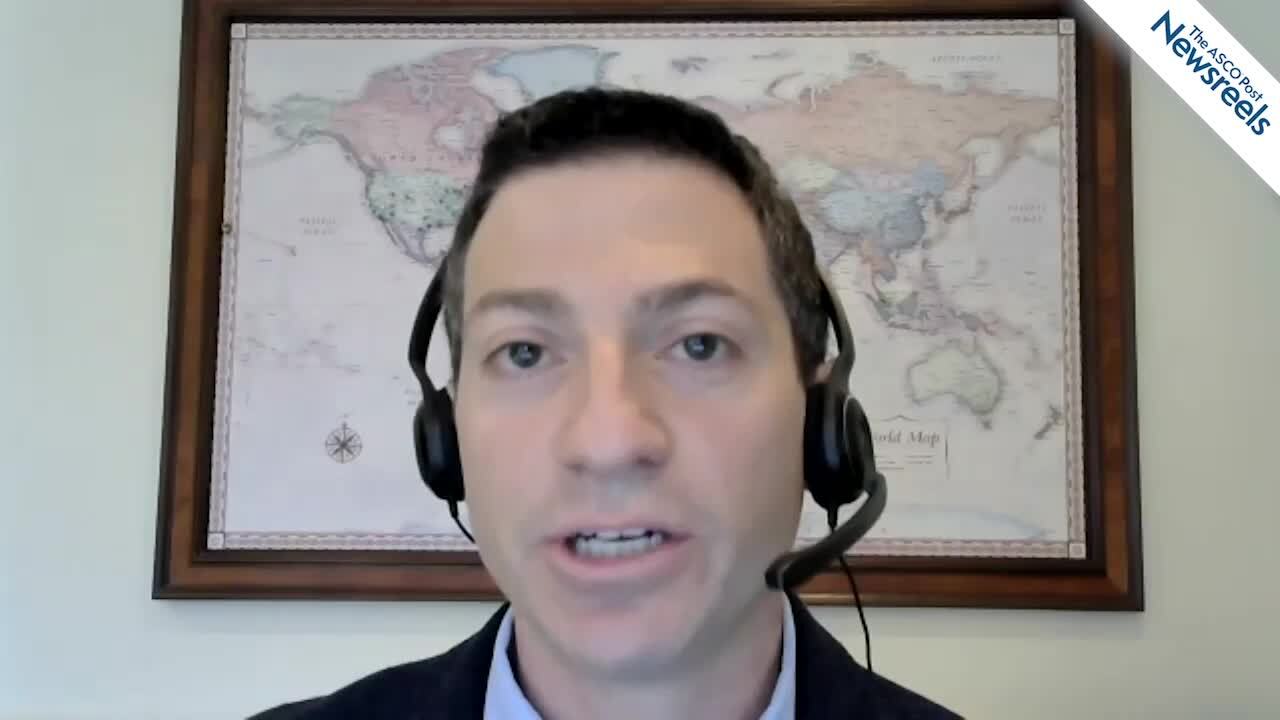
Caron A. Jacobson, MD, on Treating Large B-Cell Lymphoma With Axicabtagene Ciloleucel
2020 ASH Annual Meeting & Exposition
Caron A. Jacobson, MD, of the Dana-Farber Cancer Institute, discusses results from the ZUMA-9 C2 study, an ongoing trial that is exploring axicabtagene ciloleucel in patients with relapsed or refractory large B-cell lymphoma (Abstract 2100).
The ASCO Post Staff
Matthew S. Davids, MD, of Dana-Farber Cancer Institute, summarizes three key studies from a session he co-moderated on ibrutinib plus venetoclax for first-line treatment of patients with chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL), long-term responses to these agents for relapsed and refractory CLL, and undetectable minimal residual disease following fixed-duration treatment with venetoclax and rituximab for CLL (Abstracts 123, 124, and 125).
The ASCO Post Staff
Curtis Lachowiez, MD, of The University of Texas MD Anderson Cancer Center, discusses an interim analysis of a phase Ib/II study showing that venetoclax plus chemotherapy represents an effective regimen, particularly in patients with newly diagnosed and relapsed or refractory acute myeloid leukemia. The regimen appears to be an effective bridge to hematopoietic stem cell transplantation (Abstract 332).
The ASCO Post Staff
Nitin Jain, MD, of The University of Texas MD Anderson Cancer Center, reviews six important abstracts on CAR T-cell treatments for B-cell acute lymphoblastic leukemia (ALL): successful 24-hour manufacture of CAR T-cell therapy; ALLCAR19, a novel fast-off rate therapy; donor-derived CD19-targeted treatment; CAR 2.0 therapy to manage post-transplant relapse; UCART22, allogeneic engineered T cells expressing anti-CD22 chimeric antigen receptor; and inotuzumab ozogamicin in pediatric CD-22–positive disease (Session 614, Abstracts 159-164).
The ASCO Post Staff
Lena E. Winestone, MD, MSHP, of the University of California, San Francisco and Benioff Children’s Hospital, reviews different aspects of bias in treatment delivery, including patient selection for clinical trials; racial and ethnic disparities in survival for indolent non-Hodgkin diffuse large B-cell lymphomas; and end-of-life hospitalization of patients with multiple myeloma, as well as outcome disparities (Abstracts 207-212).
The ASCO Post Staff
Sagar Lonial, MD, of the Emory University School of Medicine, summarizes key papers presented in a session he co-moderated on how second-generation CAR T cells can be used to treat patients with multiple myeloma (Session 653).