Hossein Borghaei, DO, on Bispecific T-Cell–Engager Immune Therapy for Small Cell Lung Cancer
IASLC 2020 World Conference on Lung Cancer in Singapore
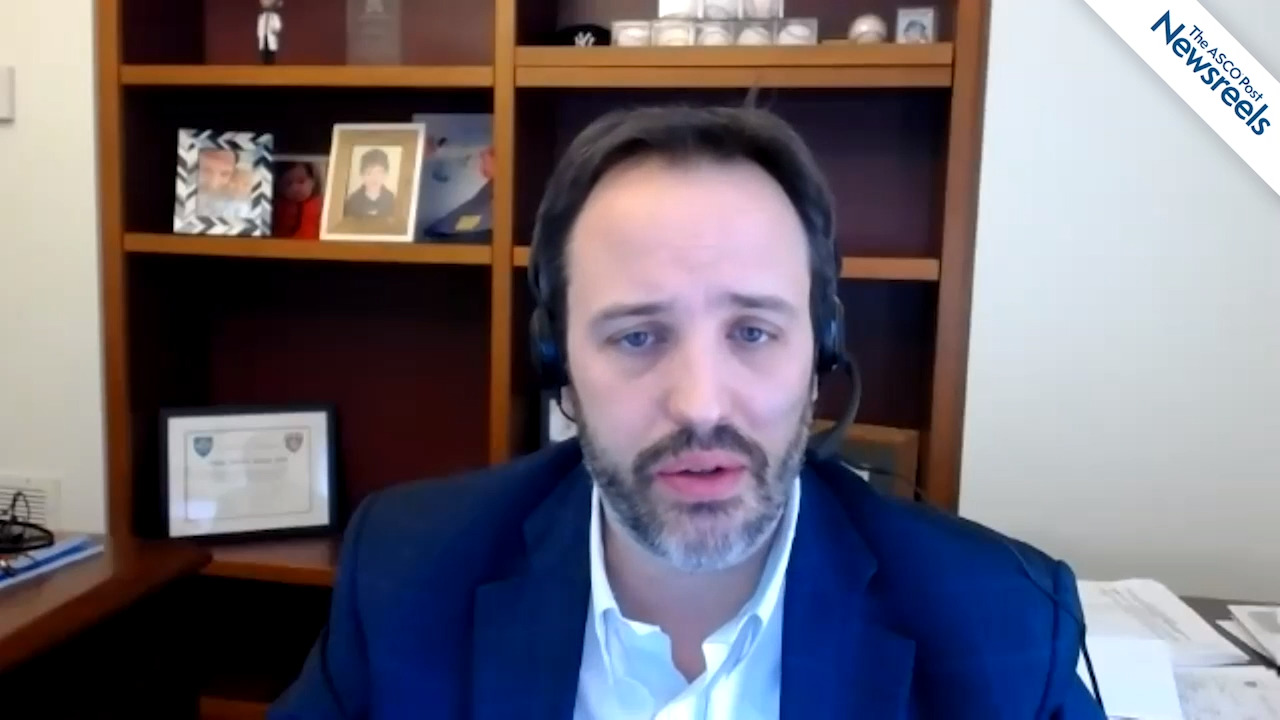
Hossein Borghaei, DO, of Fox Chase Cancer Center, discusses phase I results from a study of AMG 757, an experimental bispecific T-cell–engager (BiTE) immune therapy aimed at the DLL3 molecular target in patients with small cell lung cancer. At this early stage, results show clinical efficacy and safety, with 37% of 51 evaluable patients exhibiting disease control (Abstract OA11.03).
The ASCO Post Staff
Giorgio V. Scagliotti, MD, PhD, of the University of Torino, talks about why he believes that many more patients with lung cancer can be cured within the next 4 years, given decreases in mortality rates, widespread use of targeted treatments and immunotherapies, and earlier diagnoses as a result of systematic screening with low-dose CT (Abstract PL05.08).
The ASCO Post Staff
Martin Reck, MD, PhD, of the LungenClinic, discusses the results from KEYNOTE-799, which explored a new strategy to increase the intensity of treatment in patients with unresectable, locally advanced, stage III non–small cell lung cancer (Abstract OA02.03).
The ASCO Post Staff
Justin F. Gainor, MD, of Massachusetts General Hospital, discusses two key phase II studies on non–small cell lung cancer: nivolumab vs nivolumab plus ipilimumab in EGFR-mutant disease and the oral selective AXL inhibitor bemcentinib with pembrolizumab in advanced disease (Abstracts OA01.06 and OA01.07).
The ASCO Post Staff
Bruce E. Johnson, MD, of Dana-Farber Cancer Institute, offers his expert perspective on single-arm drug approvals for targeted agents between 2016 and 2020, the need for biomarker testing, and the societal costs of drug development (Abstract PL04.03).
The ASCO Post Staff
Silvia Novello, MD, PhD, of the University of Turin, discusses phase III results from the ITACA trial, which explored the notion of improving survival by customizing treatment and reducing toxicities for patients with completely resected stage II to IIIA non–small cell lung cancer (Abstract PS01.04).