Joseph M. Unger, PhD, on Patient Interest in Clinical Trial Enrollment: A New Analysis
2020 ASCO Quality Care Symposium
Joseph M. Unger, PhD, of Fred Hutchinson Cancer Research Center, discusses study results showing that more than half of all patients with cancer, regardless of race or ethnicity, agree to take part in clinical trials, a finding that upends conventional beliefs. He talks about removing the barriers to help more patients participate (Abstract 92).
The ASCO Post Staff
Katherine Enright, MD, MPH, of Trillium Health Partners in Ontario, discusses a model of quality improvement, which potentially could be adapted across health systems to improve oral systemic cancer care (Abstract 184).
The ASCO Post Staff
Marie A. Flannery, PhD, and Eva Culakova, PhD, both of the University of Rochester, discuss a geriatric assessment tool that helped reduce symptomatic toxicities, as measured by Patient-Reported Outcomes Common Terminology Criteria for Adverse Events (Abstract 138).
The ASCO Post Staff
Anne M. Barry-Weers, RN, of Aurora Health Care/Aurora Cancer Care in Milwaukee, discusses strategies that helped patients with cancer to better manage their chemotherapy-related symptoms at home, thus reducing visits to the emergency department and inpatient admissions, and improving treatment costs (Abstract 11).
The ASCO Post Staff
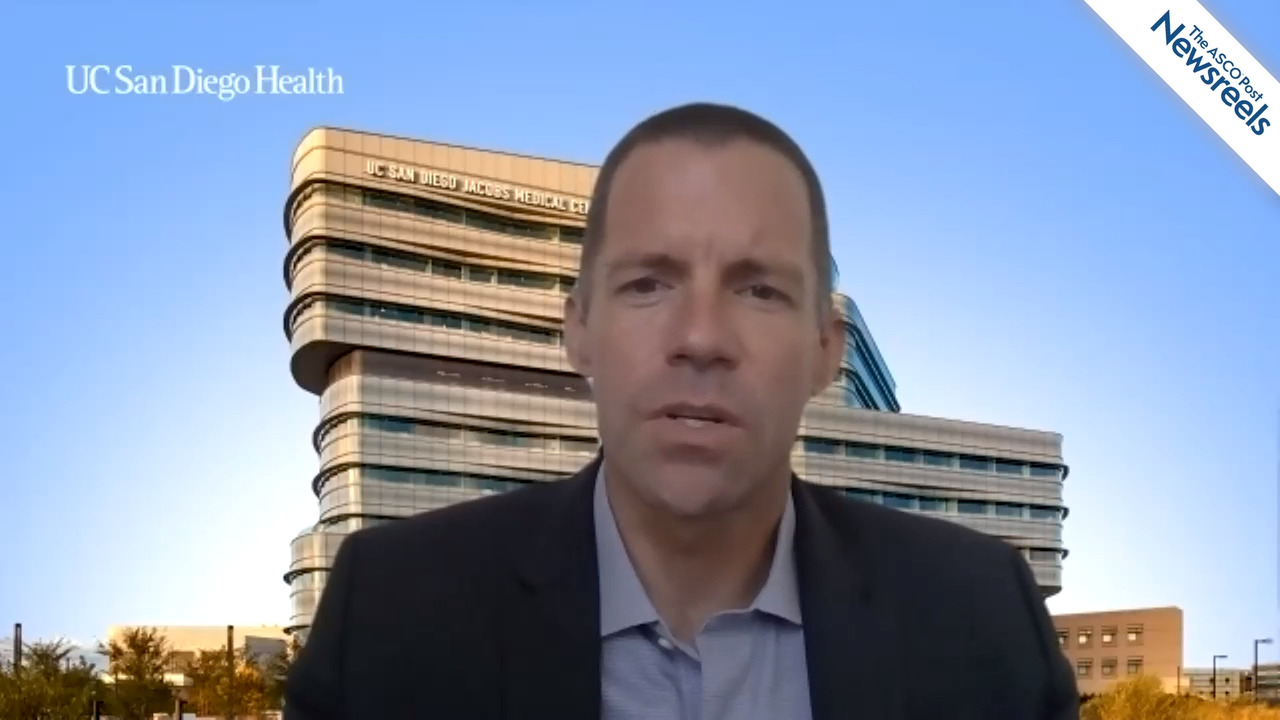
James D. Murphy, MD, of the University of California, San Diego, discusses the possible reasons for a decline in long-term opioid use in patients with cancer, even as short-term use is rising, as well as the racial and socioeconomic disparities of opioid use in this population (Abstract 187).
The ASCO Post Staff
Cardinale B. Smith, MD, PhD, of the Icahn School of Medicine at Mount Sinai, discusses findings showing Black, Hispanic, and Asian patients with cancer used telehealth less often during the COVID-19 pandemic than White patients with cancer, a negative trend that will become more problematic as this method of care continues to increase through subsequent waves of coronavirus infection and plays a larger role in standard treatment (Abstract 87).