The ASCO Post is pleased to present Hematology Expert Review, an ongoing feature that quizzes readers on issues in hematology. In this installment, Syed Ali Abutalib, MD, and L. Jeffrey Medeiros, MD, explore the impact of the prognostic marker RUNX1::RUX1T1 fusion on the diagnosis and treatment of patients with acute myeloid leukemia (AML). For each quiz question that follows, select the one best answer. The correct answers and accompanying discussions appear below.
GUEST EDITORS

Syed Ali Abutalib, MD

L. Jeffrey Medeiros, MD
Dr. Abutalib is Director of the Hematologic Malignancies, Hematopoietic Stem Cell Transplantation & Cellular Therapy Programs at the Advocate/Aurora St. Luke’s Medical Center, Milwaukee, and Associate Professor at Rosalind Franklin University of Medicine and Science, Chicago.Dr. Medeiros is Professor and Chair, Department of Hematopathology, The University of Texas MD Anderson Cancer Center, Houston.
Introduction
AML with RUNX1::RUNX1T1 is defined by a DNA fusion between RUNX1, which encodes a member of the RUNX family of transcription factors, and RUNX1T1, which encodes a transcriptional co-repressor. RUNX1 is a master transcriptional regulator of adult hematopoiesis. This genetic rearrangement is most commonly observed within the French-American-British (FAB) M2 morphologic subtype of AML.1 AML with RUNX1::RUNX1T1 is more frequently diagnosed in children than in adults and represents approximately 6% to 8% of all AML cases.1,2
Question 1
Which of the following statements regarding adults with AML harboring RUNX1::RUNX1T1 fusion is accurate?
-
- The presence of the RUNX1::RUNX1T1 fusion alone is sufficient to establish the diagnosis of AML, even when the blast percentage is less than 20%.
- This AML subtype demonstrates increased sensitivity to repeated cycles of intermediate- to high-dose cytarabine.
- Early reductions in RUNX1::RUNX1T1 transcript levels—either at the end of induction therapy or after initial consolidation cycles—are predictive of favorable outcomes with intensive chemotherapy.
- All of the above.
Question 2
Which of the following immunophenotypic findings is most commonly observed in AML with RUNX1::RUNX1T1 fusion?
- There is aberrant expression of B lymphoid markers.
- PAX5 expression is usually absent.
- CD34 expression is absent.
- Myeloperoxidase is generally absent.
Question 3
Which of the following statements about the histopathology of AML with RUNX1::RUNX1T1 fusion is correct?
- Dysplasia is absent in the bone marrow.
- Auer rods are not observed.
- Bone marrow smears show increased blasts and myeloid precursors at the promyelocyte stage.
- Eosinophil precursors are frequently increased, but they do not exhibit cytologic or cytochemical abnormalities.
Answers to Hematology Expert Review Questions
Question 1
Which of the following statements regarding adults with AML harboring RUNX1::RUNX1T1 fusion is accurate?
Correct answer: D. All of the above.
Expert Perspective
The presence of the RUNX1::RUNX1T1 fusion, resulting from the reciprocal translocation t(8;21)(q22;q22.1), is sufficient to establish a diagnosis of AML, even when the blast count is below 20%.1 This cytogenetic abnormality defines a distinct AML subtype associated with a relatively favorable prognosis, particularly in response to intermediate- to high-dose cytarabine, which is often used during consolidation therapy.
Initial RUNX1::RUNX1T1 transcript levels must be obtained at diagnosis to establish a baseline for subsequent molecular monitoring. Early molecular responses, specifically significant reductions in RUNX1::RUNX1T1 transcript levels after induction or initial consolidation, are strong predictors of improved relapse-free survival.3,4 Among various prognostic indicators, measurable residual disease (MRD) status—particularly as assessed via fusion transcript levels—has emerged as the most powerful predictor of long-term outcomes, surpassing the prognostic value of other adverse markers such as KIT mutations, high white blood cell count, CD56 expression, myeloid sarcoma, and/or additional cytogenetic abnormalities in adults.2-5
In adults with AML harboring RUNX1::RUNX1T1 fusion, MRD should be evaluated at specific intervals, as indicated here5:
- In peripheral blood after two cycles of chemotherapy;
- In bone marrow at the completion of consolidation therapy;
- In peripheral blood every 4 to 6 weeks for up to 2 years after consolidation, to monitor for potential relapse.
Question 2
Which of the following immunophenotypic findings is most commonly observed in AML with RUNX1::RUNX1T1 fusion?
Correct answer: A. There is aberrant expression of B lymphoid markers.
Expert Perspective
Leukemic cells in AML with RUNX1::RUNX1T1 fusion typically exhibit a distinct immunophenotypic profile.1 This profile includes high-intensity expression of CD34 and aberrant expression of B lymphoid markers, such as CD19, PAX5, and cytoplasmic CD79a. Additional markers frequently expressed include HLA-DR, CD13, and myeloperoxidase, whereas CD33 tends to be weak or absent. The blasts often display maturation asynchrony, such as co-expression of CD34 and CD15 (immature myeloid cells with partial maturation).
Question 3
Which of the following statements about the histopathology of AML with RUNX1::RUNX1T1 fusion is correct?
Correct answer: D. Eosinophil precursors are frequently increased, but they do not exhibit cytologic or cytochemical abnormalities.
Expert Perspective
Bone marrow trephine biopsy (Figure 1A) or clot sections typically reveal extensive replacement by sheets of immature cells. Bone marrow aspirate smears (Figure 1B) demonstrate increased blasts and myeloid precursors at various stages of maturation in approximately 90% of cases, whereas about 10% show features consistent with AML without maturation.1
The blasts are generally large, with abundant basophilic cytoplasm, often containing numerous azurophilic granules and perinuclear clearing (cytoplasmic hofs). Occasionally, blasts may contain large Chediak-Higashi–like granules. Auer rods are commonly observed, usually appearing as single, elongated structures with tapered ends.
Promyelocytes, myelocytes, and mature neutrophils often exhibit dysplastic features, including abnormal nuclear segmentation (such as pseudo–Pelger-Huët nuclei) and cytoplasmic staining abnormalities (eg, homogeneous pink cytoplasm in neutrophils). However, dysplasia in other hematopoietic lineages is generally uncommon. Monocytes are typically scarce or absent.
Eosinophil precursors are frequently increased, but they characteristically lack cytologic or cytochemical abnormalities. Basophils may also be elevated. In some cases, mast cells are increased, and rarely, concurrent systemic mastocytosis may be present.1
DISCLOSURE: Dr. Abutalib has served on the advisory board of AstraZeneca. Dr. Medeiros reported no conflicts of interest.
REFERENCES
- Alaggio R, Amador C, Anagnostopoulos I, et al: World Health Organization Classification of Haematolymphoid Tumours: Acute Myeloid Leukaemia With RUNX1::RUNX1T1 Fusion, 5th ed, pp 278-283. Lyon, France; International Agency for Research on Cancer; 2025.
- Borthakur G, Kantarjian H: Core binding factor acute myelogenous leukemia: 2021 Treatment algorithm. Blood Cancer J 11:114, 2021.
- Heuser M, Freeman SD, Ossenkoppele GJ, et al: 2021 Update on MRD in acute myeloid leukemia: A consensus document from the European LeukemiaNet MRD Working Party. Blood 138:2753-2767, 2021.
- Rücker FG, Agrawal M, Corbacioglu A, et al: Measurable residual disease monitoring in acute myeloid leukemia with t(8;21)(q22;q22.1): Results from the AML Study Group. Blood 134:1608-1618, 2019.
- Zhu HH, Zhang XH, Qin YZ, et al: MRD-directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: Results from the AML05 multicenter trial. Blood 121:4056-4062, 2013.
Dr. Abutalib is Director of the Hematologic Malignancies, Hematopoietic Stem Cell Transplantation & Cellular Therapy Programs at the Advocate/Aurora St. Luke’s Medical Center, Milwaukee, and Associate Professor at Rosalind Franklin University of Medicine and Science, Chicago. Dr. Medeiros is Professor and Chair, Department of Hematopathology, The University of Texas MD Anderson Cancer Center, Houston.
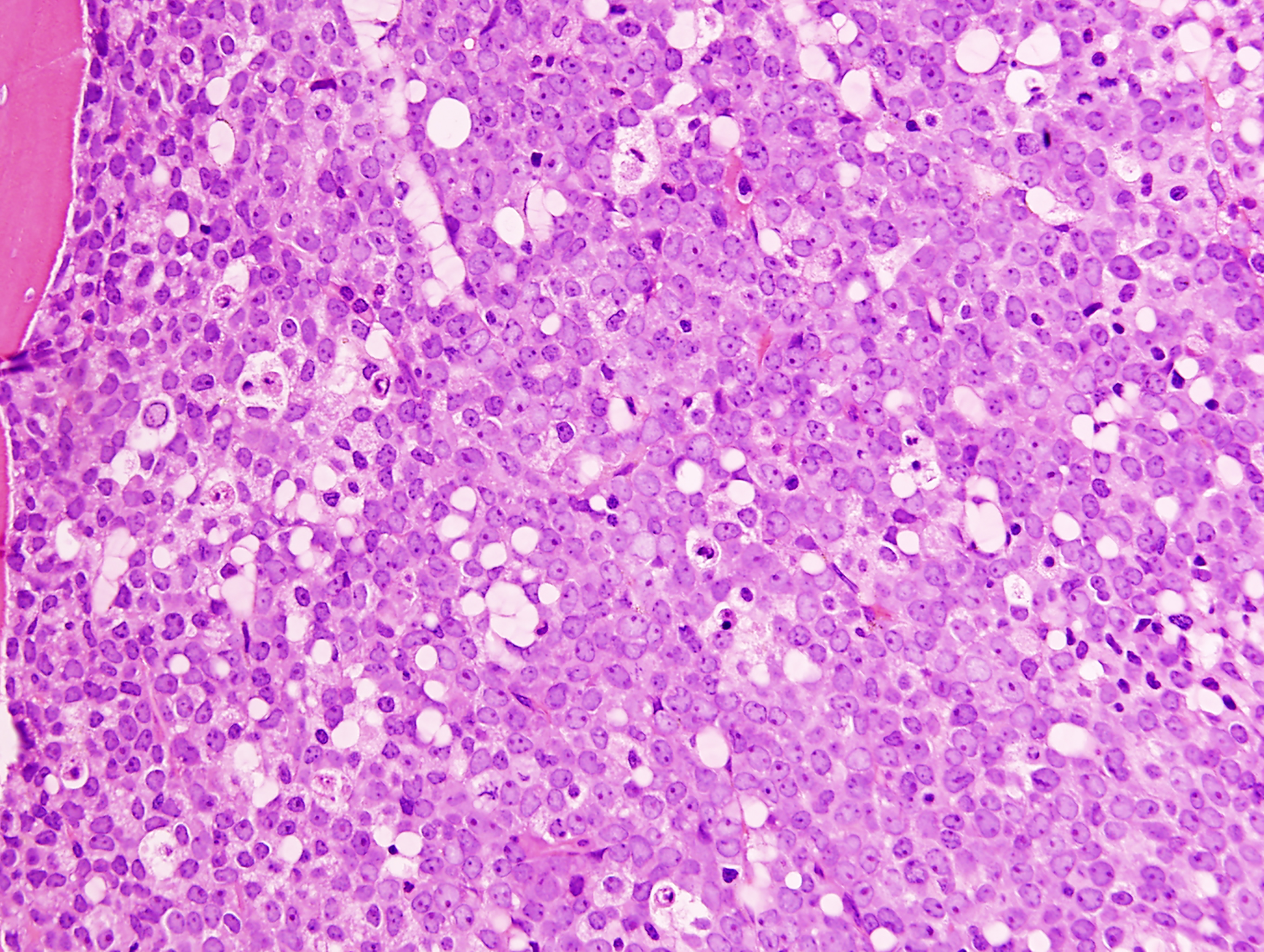
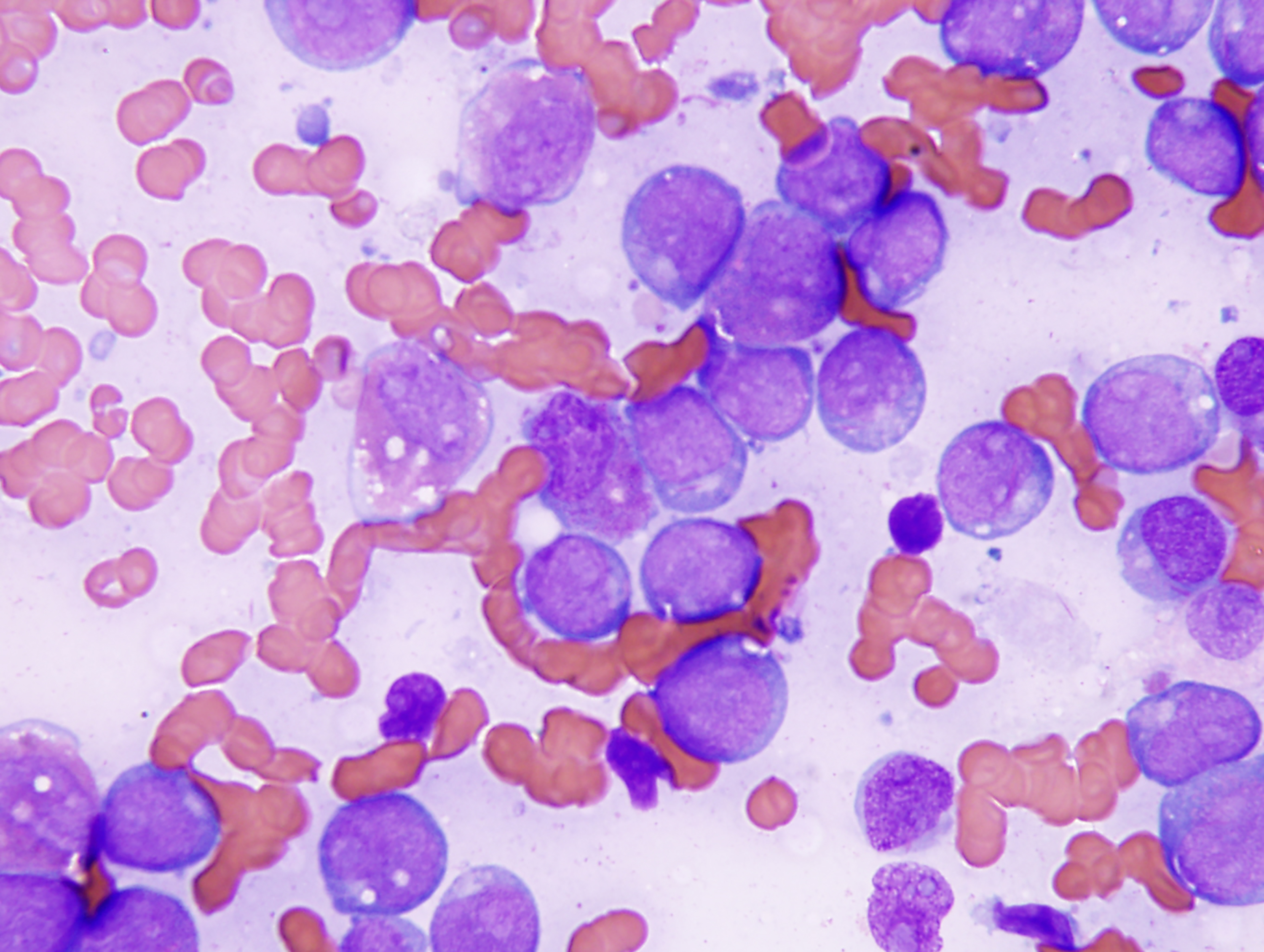
FIGURE 1 (Image A [TOP], Image B [BOTTOM]): Acute myeloid leukemia with RUNX1::RUNX1T1 involving the bone marrow. A. The biopsy specimen shows diffuse replacement of the medullary space by blasts. Trabecular bone is present in the top left corner of this image. B. The aspirate smear shows many blasts with perinuclear cytoplasmic clearing and a few abnormal, maturing granulocytic cells in the background. Three blasts in this image contain thin Auer rods in their cytoplasm.

