
Stuart M. Lichtman, MD, FACP, FASCO
The aging population, now the largest group of patients with cancer and cancer survivors, requires a rigorous and focused approach to clinical trial reporting, a need highlighted by the recent author guidelines from the Journal of Clinical Oncology (JCO; Table 1).1 The guidelines are an important first step to improve the reporting of clinical trials, a recommendation long sought by the geriatric oncology community, and it will be gratifying to see them implemented.2-4
I want to commend the JCO for these recommendations on the reporting of clinical trials. The JCO is the first journal to acknowledge the significance and urgent need for this issue.
Their adoption will undoubtedly enhance the care of older patients, leading to more tailored and safer treatment protocols. Older patients deserve nothing less than to be the central focus of our efforts. Oncology researchers and clinicians are, in essence, all geriatric oncologists; these guidelines will encourage that realization and foster a more patient-centered approach to cancer care.
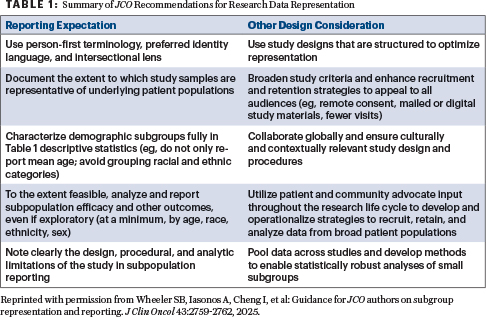
However, I believe these guidelines could have gone further regarding studies of older patients. It is crucial for clinicians to effectively use trial information for patient care. Given that clinical trial patients often do not fully represent the general population, a detailed statistical breakdown offers vital insights into patient care strategies for the older patient population, who are chronically underrepresented in clinical trials. ASCO position papers have consistently highlighted this issue, emphasizing the need for greater inclusivity and detailed analysis concerning this demographic.5-7 (For more on the first ASCO guideline on cancer-specific geriatric assessment of older patients, see here.)
Comprehensive Reporting: An Ethical Imperative
Because of the low enrollment of older patients in clinical trials, relevant data are frequently scarce. However, greater emphasis is urgently needed on this population. When subgroup analysis is performed, specific questions must be addressed with rigorous detail: Did older patients benefit as much as younger patients? Was their toxicity profile greater, and if so, in which specific ways? Which subgroup experienced the most toxicity, and what were the defining characteristics of those patients? If a study reports a percentage of grade 3 or 4 toxicity, the demographic, comorbidity, and functional status of those specific patients should be thoroughly detailed to provide clinical context. Furthermore, even grade 2 toxicity can adversely impact quality of life and cause functional decline. Also, did therapy maintain, improve, or decrease function?
In older patients, this information should be robustly reported, as it adds significant clinical benefit without increasing research cost. Such comprehensive reporting is an ethical imperative; without it, patients may be exposed to unnecessary toxicity due to a lack of precise understanding of treatment effects in their specific demographic.
Geriatric Baseline Parameters: A Critical Need
Clinical trial design must integrate geriatric baseline parameters, aligning with ASCO guidelines and modified templates from the Cancer Therapy Evaluation Program (CTEP), which have evolved to recognize this critical need. Without these specific parameters, such as assessments of functional status, cognitive function, nutritional status, and comorbidity, data on how treatments affect older individuals remain incomplete. This information gap obscures whether older patients derive comparable benefits to younger patients. It increases the risk of exposing older patients to avoidable toxicity, given their unique responses to treatments, such as greater toxicity or impact on quality of life. Therefore, comprehensive reporting of these parameters is essential for the ethical conduct of clinical trials and for developing safer, more effective, and truly personalized treatment strategies for the older population.8
Reviewers, including institutional review boards, the National Cancer Institute, and other relevant groups, should mandate the collection and reporting of these data in all appropriate studies. These JCO guidelines should be required, and if a study fails to meet these standards, editors should demand a written explanation, published alongside the paper, outlining the reasons for noncompliance and any plans for future data collection.
The JCO and other journals must require these guidelines immediately and further expand them as noted. I am gratified that the JCO editors took up the challenge I proposed in a compelling e-mail back in September 2023. Without it, maybe these guidelines would not have come to pass.
DISCLOSURE: Dr. Lichtman reported no conflicts of interest.
REFERENCES
1. Wheeler SB, Iasonos A, Cheng I, et al: Guidance or JCO authors on subgroup representation and reporting. J Clin Oncol 43:2759-2762, 2025.
2. Lichtman SM: Call for changes in clinical trial reporting of older patients with cancer. J Clin Oncol 30:893-894, 2012.
3. Lichtman SM: Missed opportunities in geriatric oncology research. Oncologist 28:373-375, 2023.
4. Lichtman SM: All oncologists are geriatric oncologists...they just don’t know it yet. The ASCO Post. August 25, 2019.
5. Dale W, Klepin HD, Williams GR, et al: Practical assessment and management of vulnerabilities in older patients receiving systemic cancer therapy: ASCO guideline update. J Clin Oncol 41:4293-4312, 2023.
6. Mohile SG, Dale W, Somerfield MR, et al: Practical assessment and management of vulnerabilities in older patients receiving chemotherapy. ASCO guideline for geriatric oncology. J Clin Oncol 36:2326-2347, 2018.
7. Hurria A, Levit LA, Dale W, et al: Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology statement. J Clin Oncol 33:3826-3833, 2015.
8. DuMontier C, Dale W, Revette AC, et al: Ethics of overtreatment and undertreatment in older adults with cancer. BMC Med Ethics 26:105, 2025.
Dr. Lichtman is an attending physician (retired) at Memorial Sloan Kettering Cancer Center, Commack, New York; Professor of Medicine at Weill Cornell Medical College, New York; a consultant for Wilmot Cancer Institute Geriatric Oncology Research, University of Rochester; and Past President of the International Society of Geriatric Oncology (SIOG). Dr. Lichtman is also Guest Editor of the Geriatrics for the Oncologist column in The ASCO Post.
Disclaimer: This commentary represents the views of the author and may not necessarily reflect the views of ASCO or The ASCO Post.

