With the introduction of tyrosine kinase inhibitors, chronic myeloid leukemia (CML) has become a chronic disease for most patients. Furthermore, we now know that some patients can successfully stop treatment and remain in remission.
“Stopping tyrosine kinase inhibitor therapy is something we get a lot of questions about from both patients and their referring physicians,” said Amelia A. Langston, MD, Professor of Hematology and Medical Oncology at Emory University School of Medicine, Atlanta. Although their efficacy is indisputable, the tradeoff can be toxicities, cost, and the psychological burden of being on medication potentially for a lifetime, she said during a presentation at the 2024 Debates and Didactics in Hematology and Oncology conference, sponsored by Emory University.1

Amelia A. Langston, MD
Discontinuation of tyrosine kinase inhibitors is feasible in some patients who achieve a sustained deep molecular response, which is a four-log reduction in BCR::ABL transcripts (≤ 0.01% on an international reporting scale [IS], or MR4). Dr. Langston posed these questions: Which patients are good candidates for treatment discontinuation, how should they be monitored, and how should relapses after discontinuation be treated?
Studies in Support of Treatment Discontinuation
The first study to document the safety of discontinuation of tyrosine kinase inhibitors, STIM1 (Stop Imatinib), involved 100 patients for whom imatinib induced a sustained deep molecular response for at least 2 years.2 The molecular recurrence-free survival rate was 43% at 6 months and 38% at 60 months, with the latest relapse occurring at 19 months. All relapsing patients responded to reintroduction of imatinib.
These findings ushered in a number of studies of the second-generation agents, which also demonstrated that a substantial subset of patients achieving a sustained deep molecular response could successfully stop therapy. The largest trial of treatment discontinuation was EURO-SKI; in this study, discontinuation of tyrosine kinase inhibitor therapy was evaluated in 758 patients who had been on treatment at least 3 years (none with treatment failure) and had sustained at least MR4 for at least 1 year.3 Of note, Dr. Langston said, the definition of failure was not relapse but MR3. “Patients can return to low levels of transcripts without overt disease recurrence in some cases,” she explained.
The molecular recurrence-free survival rate was 61% at 6 months and 46% at 36 months. At 12 months, 92% of patients who lost major molecular response and restarted a tyrosine kinase inhibitor demonstrated recovery of sustained deep molecular response. The predictors of maintaining a major molecular response at 6 to 36 months were duration of tyrosine kinase inhibitor therapy and duration of molecular response, with each additional year of sustained deep molecular response offering a 3% increase in the odds of remaining in response. For late losses of major molecular response after 6 months, the duration of tyrosine kinase inhibitor treatment, the percentage of blasts in peripheral blood, and the platelet count at diagnosis were significant factors. This suggests that early (< 6 months) and later relapses may be biologically different, she noted.
Numerous studies document that some patients have stable low-level persistence of BCR::ABL transcripts without clinical relapse after discontinuation of tyrosine kinase inhibitors. There is also some signal of persistence in lymphocytes and in nonproliferating neoplastic cells associated with leukemia stem cell exhaustion. Evidence is also emerging that the immune system may play a role in maintaining treatment-free remission.
Recommendations for a Treatment Discontinuation Trial
“A significant fraction of patients, but certainly not all, may be eligible for a tyrosine kinase inhibitor discontinuation trial,” Dr. Langston said. She noted that patients receiving a second-generation tyrosine kinase inhibitor are more likely to attain the necessary sustained deep molecular response and to do so sooner than those receiving imatinib.
Two sets of guidelines—one from the National Comprehensive Cancer Network® (NCCN®) and one from the European LeukemiaNet (ELN)4—pertain to discontinuation of tyrosine kinase inhibitors (Table 1).
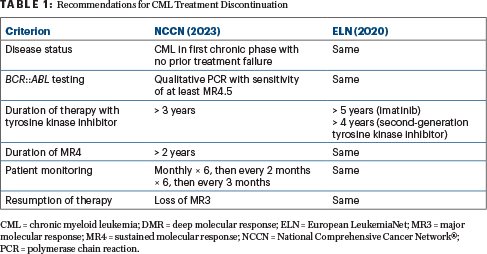
Both recommend a discontinuation trial only in patients with CML in first chronic phase with no prior treatment failure. Both require high-sensitivity quantitative polymerase chain reaction and quick return of results. The NCCN recommendations are more lenient as to the duration of tyrosine kinase inhibitor therapy. The most critical thing is to closely monitor patients for resumption of MR3 loss, she said. Patients should be monitored monthly for the first 6 months, since most relapses occur early after treatment discontinuation; beyond 6 months, monitoring at 3-month intervals is sufficient.
One special situation in which discontinuation of treatment is recommended is pregnancy. “If you’ve taken care of patients who get pregnant while on a tyrosine kinase inhibitor, you know there is a bit of hand wringing, particularly if the patient is not in a sustained deep molecular response at the desired time of treatment discontinuation,” stated Dr. Langston. The risk is greatest in the first trimester, but the consensus is that tyrosine kinase inhibitors should be stopped throughout pregnancy.
“If the patient is in deep molecular remission, it’s best to stop treatment prior to the attempt to conceive, with monthly monitoring. For patients in chronic phase who are not in deep molecular remission, monthly monitoring is still appropriate. For patients beyond chronic phase CML, a frank discussion is in order, as maintaining disease control without a tyrosine kinase inhibitor or chemotherapy is less likely,” she said. If treatment is necessary—when patients lose hematologic control—interferon is safe during pregnancy.
Tyrosine Kinase Inhibitor Withdrawal Syndrome
“Tyrosine kinase inhibitor withdrawal syndrome occurs in a significant minority of patients (10%–40%) after stopping the tyrosine kinase inhibitor, typically early after stopping the drug and more frequently in women,” Dr. Langston explained. This syndrome is often manifest by worsening musculoskeletal pain, with or without a rash. Risk of developing this syndrome seems related to longer duration of tyrosine kinase inhibitor treatment and antecedent arthritis symptoms. Tapering the tyrosine kinase inhibitor seems to have little effect on its incidence or severity, she added.
Management of tyrosine kinase inhibitor withdrawal syndrome is symptomatic. A brief course of steroids may be useful in severe cases. Restarting the tyrosine kinase inhibitor is not necessary, and symptoms generally resolve within a few months.
What About Patients Who Do Not Respond to Initial Treatment Discontinuation?
Numerous small studies indicate that a second attempt at tyrosine kinase inhibitor discontinuation can be successful in some patients who achieve a second sustained deep molecular response. “We don’t necessarily know who those patients are,” acknowledged Dr. Langston.
Time to molecular relapse after the first attempt is most predictive of a successful second attempt, with several studies showing relatively favorable results if the molecular relapse was at least 3 months after initial discontinuation of the tyrosine kinase inhibitor. This means, she said, “patients with really early relapses are probably not great candidates for a second attempt, but there are no current consensus recommendations regarding this.”
Dr. Langston continued: “For relapsing patients, tyrosine kinase inhibitors need to be resumed quickly. Get them back to at least MR3 after treatment failure. It’s not unreasonable to think about a second discontinuation attempt down the road but enroll these patients on a clinical trial. We need to learn more about how to select patients for a second attempt at treatment discontinuation,” she added.
DISCLOSURE: Dr. Langston reported no conflicts of interest.
REFERENCES
2. Etienne G, Guilhot J, Rea D, et al: Long-Term Follow-Up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol 35:298-305, 2017.
3. Mahon FX, Pfirrmann M, Dulucq S, et al: European Stop Tyrosine Kinase Inhibitor Trial (EURO-SKI) in chronic myeloid leukemia: Final analysis and novel prognostic factors for treatment-free remission. J Clin Oncol 42:1875-1880, 2024.
4. Hochhaus A, Baccarani M, Silver RT, et al: European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 34:966-984, 2020.

