 Molecular diversity—its existence, extent, and implications for therapy—was a central theme of key metastatic lung cancer studies presented at this year’s ASCO meeting, according to D. Ross Camidge, MD, PhD, of the University of Colorado, Denver, who addressed major findings in advanced lung cancer at the Best of ASCO Seattle meeting.
Molecular diversity—its existence, extent, and implications for therapy—was a central theme of key metastatic lung cancer studies presented at this year’s ASCO meeting, according to D. Ross Camidge, MD, PhD, of the University of Colorado, Denver, who addressed major findings in advanced lung cancer at the Best of ASCO Seattle meeting.
Adenocarcinomas Are Highly Molecularly Diverse
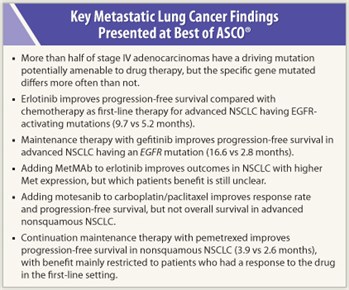
More than half of lung adenocarcinomas have a mutation driving the cancer that is potentially amenable to drug therapy, but the specific gene mutated differs more often than not, finds a study by the National Cancer Institute’s Lung Cancer Mutation Consortium.1
The investigators assessed 10 molecular markers in 1,000 stage IV adenocarcinomas. Of the first 516 undergoing full molecular analysis, 54% had a so-called actionable mutation—ie, one with a targeting drug available or in development (Fig. 1). Mutations were found in all 10 genes, with the majority in the genes for KRAS (22% of all tumors studied), EGFR (17%), and EML4-ALK (7%).
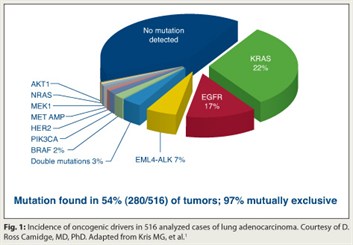 Additionally, in 97% of these tumors, there was only a single driving actionable mutation. The rare double mutations were usually related to PI3 kinase.
Additionally, in 97% of these tumors, there was only a single driving actionable mutation. The rare double mutations were usually related to PI3 kinase.
“Now the question you may be asking yourself is, do these represent the lung adenocarcinomas which I see in my practice?” Dr. Camidge noted. Patient demographics presented subsequently2 suggest they don’t: 59% of the patients were female, the median age was 60, and 31% were never-smokers.
“Although exact proportions [of patients] in your clinic may differ from [data compiled by the Consortium], I still think you will agree that we are starting to clearly understand that lung adenocarcinoma is not a single disease on the molecular level,” he maintained.
Erlotinib vs Chemotherapy in NSCLC with EGFR Mutations
The targeted agent erlotinib (Tarceva) is more efficacious than chemotherapy as first-line therapy for advanced NSCLC having activating mutations of the epidermal growth factor receptor (EGFR), according to interim results of the phase III EURTAC trial.3
A total of 174 patients were randomly assigned to platinum-based doublet chemotherapy or erlotinib, an EGFR tyrosine kinase inhibitor. Patients in the erlotinib group had better progression-free survival (9.7 vs 5.2 months; HR = 0.37; P < .0001) and a better objective response rate (58% vs 15%).
 Overall survival did not differ significantly between groups, but the data are not mature. Additionally, most patients given chemotherapy who had disease progression received second-line therapy—usually an EGFR tyrosine kinase inhibitor. “So I would be very surprised if overall survival is likely to be positive in this study,” Dr. Camidge commented.
Overall survival did not differ significantly between groups, but the data are not mature. Additionally, most patients given chemotherapy who had disease progression received second-line therapy—usually an EGFR tyrosine kinase inhibitor. “So I would be very surprised if overall survival is likely to be positive in this study,” Dr. Camidge commented.
The take-home message here? “If you are known to have an EGFR mutation in time to make a first-line treatment decision, then it would appear that progression-free survival and objective response rate are far better if you get an EGFR tyrosine kinase inhibitor than chemotherapy,” he said.
Results of this study, conducted in Europe, are consistent with those conducted in Asia, Dr. Camidge added. This suggests “that it’s the tumor’s molecular biology and not the host’s molecular biology which is really the dominant driver of results. That is, an EGFR-mutant Asian patient is very similar to an EGFR-mutant Caucasian patient.”
Switch Maintenance Therapy with Gefitinib
The phase III INFORM trial found that switch maintenance therapy (immediate non–cross resistant alternative therapy) with the targeted agent gefitinib (Iressa) is efficacious in patients with locally advanced or metastatic NSCLC.4
Patients in China were eligible for the trial if they had completed four cycles of first-line platinum-based chemotherapy without progressive disease or unacceptable toxicity. In all, 296 patients were randomly assigned to gefitinib (another EGFR tyrosine kinase inhibitor) or placebo.
The rate of progression-free survival was better with gefitinib in the entire trial population (median, 4.8 vs 2.6; HR = 0.42; P < .0001). Only a quarter of patients had tissue available for EGFR mutation status, of whom 38% had an EGFR mutation. Gefitinib improved progression-free survival in patients with an EGFR mutation (16.6 vs 2.8 months; HR = 0.17) but not in those without one.
“When you start using an EGFR tyrosine kinase inhibitor in trials, you cannot ignore the idea of EGFR mutation anymore,” Dr. Camidge stressed. Furthermore, “you cannot apply information obtained in a group where you don’t know the mutation-specific status to [a patient] for whom you increasingly do know the status. Unknown status is completely useless.”
A second option for switch maintenance, erlotinib, was tested in the SATURN trial, which also showed greater benefit among patients with EGFR mutations.5 And a third option, pemetrexed (Alimta), appears efficacious for treating nonsquamous tumors that may or may not have an EGFR mutation,6 although more-detailed analyses are still needed.
MetMAb Trial Suggests Benefit
Adding the investigational agent MetMAb to erlotinib improves outcomes in patients with advanced NSCLC whose tumors show higher expression of Met, the phase II OAM4558g trial concluded.7
A total of 137 patients needing second- or third-line therapy were randomly assigned to erlotinib plus either placebo or MetMAb, an antibody that blocks binding of hepatocyte growth factor to Met. Amplification of Met is known to be a key mechanism of resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant tumors.
Adding MetMAb did not improve outcomes in the trial population as a whole. But among patients falling into a so-called Met diagnostic-positive group, identified by immunohistochemistry, adding MetMAb improved both progression-free survival (2.9 vs 1.5 months; HR = 0.53; P = .04) and overall survival (12.6 vs 3.8 months; HR = 0.37; P = .002). Among Met diagnostic-negative patients, adding MetMAb appeared to worsen both outcomes. The findings have prompted a similar phase III trial restricted to Met diagnostic-positive patients.
“We have lots of unanswered questions about who is really benefiting,” Dr. Camidge commented. He expressed concerns about the trial’s methodology, including use of MetMAb in combination therapy without obvious justification, the lack of a biologic rationale for selecting the cutpoint for the Met diagnostic-positive group, and marked imbalance in EGFR mutations within each diagnostic group that could have explained observed differences.
Finally, in stratified analyses of EGFR mutation status, overall survival in the Met diagnostic-positive group was shown only for nonmutant tumors. “We don’t know that [those with mutant tumors] benefit from this combination, so I don’t see data justifying keeping them in the phase III study beyond preserving trial size and potential market size,” Dr. Camidge said.
Motesanib in Advanced Nonsquamous NSCLC
The phase III MONET1 trial showed that adding the investigational drug motesanib to carboplatin/paclitaxel therapy improves response rate and progression-free survival but not overall survival in patients with advanced nonsquamous NSCLC.8
A total of 1,090 patients were randomly assigned to chemotherapy plus either placebo or motesanib, an oral inhibitor of the vascular endothelial growth factor (VEGF) receptors 1, 2, and 3, platelet-derived growth factor (PDGF) receptor, and Kit. Compared with their counterparts in the placebo group, patients in the motesanib group had a higher response rate (40% vs 26%) and progression-free survival (HR = 0.79; P = .0006), but not overall survival.
“This is another antiangiogenic [tyrosine kinase inhibitor] disappointment,” Dr. Camidge commented, and one that is as yet without explanation.
“Clearly, there is desperate need to find the group of people who may or may not benefit from these [agents], and maybe we are looking in the wrong place,” he proposed. “Maybe we need to start to look in the host because it is the host vasculature which is actually climbing into the tumor.”
Maintenance with Pemetrexed in Responsive Tumors
The phase III PARAMOUNT trial finds that patients with advanced nonsquamous NSCLC who have completed pemetrexed-containing first-line therapy with at least stable disease fare better if they continue to receive pemetrexed for maintenance.9 But benefit is greatest for those who had a response to the drug in the first-line setting.
The 539 patients in PARAMOUNT were assigned 2:1 to receive best supportive care plus either continued pemetrexed or placebo. Progression-free survival was superior with pemetrexed (3.9 vs 2.6 months; HR = 0.64; P = .0002). But patients in that group had higher rates of grade 3/4 fatigue (4.2% vs 0.6%), anemia (4.5% vs 0.6%), and neutropenia (3.6% vs 0%). Overall survival data are still immature.
Dr. Camidge questioned the clinical significance of the 1.3-month gain in progression-free survival and noted subgroup analyses showed that benefit was mainly restricted to patients who had a partial or complete response to the drug in first-line therapy. “So the bottom line of whom to give pemetrexed to in the maintenance setting is the people who were already benefiting from pemetrexed,” he said.
“Now, stable disease is a very broad category,” Dr. Camidge added. “I would be very interested in seeing a waterfall plot [by which we might distinguish] the people who were minor responders and may be deriving benefit, from those who were actually slowly progressing.”
Recent data suggest that certain biomarkers, such as ALK positivity, may also help identify the patients most likely to benefit from pemetrexed.10 “I don’t know the reason for that,” he said, “but it gets back to the idea that when you molecularly fragment your group of patients with lung cancer, you can start to see patterns that you never knew existed before.” ■
Disclosure: Dr. Camidge has received honoraria from Pfizer, OSI, and AstraZeneca for ad hoc advisory boards and research funding from Lilly.
SIDEBAR: Molecular Heterogeneity Complicates Management of Resistance
References
1. Kris MG, Johnson BE, Kwiatkowski DJ, et al: Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: The NCI’s Lung Cancer Mutation Consortium (LCMC). 2011 ASCO Annual Meeting. Abstract CRA7506. Presented June 5, 2011.
2. Johnson BE, Kris MG, Kwiatkowski D, et al: Clinical characteristics of planned 1000 patients with adenocarcinoma of lung (ACL) undergoing genomic characterization in the US Lung Cancer Mutation Consortium (LCMC). 14th World Conference on Lung Cancer. Abstract 16.01. Presented July 5, 2011.
3. Rosell R, Gervais R, Vergnenegre A, et al: Erlotinib versus chemotherapy (CT) in advanced non-small cell lung cancer (NSCLC) patients (p) with epidermal growth factor receptor (EGFR) mutations: Interim results of the European Erlotinib Versus Chemotherapy (EURTAC) phase III randomized trial. 2011 ASCO Annual Meeting. Abstract 7503. Presented June 5, 2011.
4. Zhang L, Shenglin M, Song X, et al: Efficacy, tolerability, and biomarker analyses from a phase III, randomized, placebo-controlled, parallel group study of gefitinib as maintenance therapy in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC; INFORM; C-TONG 0804). 2011 ASCO Annual Meeting. Abstract LBA7511. Presented June 5, 2011.
5. Cappuzzo F, Ciuleanu T, Stelmakh L, et al: Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: A multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 11:521-529, 2010.
6. Ciuleanu T, Brodowicz T, Zielinski C, et al: Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: A randomised, double-blind, phase 3 study. Lancet 374:1432-1440, 2009.
7. Spigel DR, Ervin TJ, Ramlau R, et al: Final efficacy results from OAM4558g, a randomized phase II study evaluating MetMAb or placebo in combination with erlotinib in advanced NSCLC. 2011 ASCO Annual Meeting. Abstract 7505. Presented June 5, 2011.
8. Scagliotti G, Vynnychenko I, Ichinose Y, et al: An international, randomized, placebo-controlled, double-blind phase III study (MONET1) of motesanib plus carboplatin/paclitaxel (C/P) in patients with advanced nonsquamous non-small cell lung cancer (NSCLC). 2011 ASCO Annual Meeting. Abstract LBA7512. Presented June 6, 2011.
9. Paz-Ares LG, De Marinis F, Dediu M, et al: PARAMOUNT: Phase III study of maintenance pemetrexed (pem) plus best supportive care (BSC) versus placebo plus BSC immediately following induction treatment with pem plus cisplatin for advanced nonsquamous non-small cell lung cancer (NSCLC). 2011 ASCO Annual Meeting. Abstract CRA7510. Presented June 5, 2011.
10. Camidge DR, Kono SA, Lu X, et al: Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol 6:774-780, 2011.

