In locally advanced non–small cell lung cancer (NSCLC), immunotherapy consolidation with the PD-L1 inhibitor durvalumab improved overall survival in the PACIFIC trial,1 thus leading to its use after chemoradiotherapy as a standard of care. Real-world evidence of durvalumab’s effect on overall and progression-free survival, however, has been limited, as have assessments of treatment patterns after completion or discontinuation of durvalumab. A look at these outcomes was the aim of three studies from outside the United States, which were presented at the International Association for the Study of Lung Cancer (IASLC) 2022 World Conference on Lung Cancer.2-4
Study From Brazil

Pedro Aguiar, Jr, MD, PhD
Pedro Aguiar, Jr, MD, PhD, Assistant Professor at the Faculdade de Medicina do ABC in Brazil, presented findings from 177 patients treated at the largest private community oncology practice in Brazil.2 Overall survival rates were comparable to those shown in the PACIFIC trial. The study also demonstrated the limited effectiveness of existing salvage therapy, Dr. Aguiar said.
The 177 patients had been diagnosed with stage III NSCLC and had received durvalumab, with a median follow-up of 12 months. Researchers extracted patient-level information from an integrated multicenter database. The study’s primary endpoint was median overall survival, and secondary endpoints were the time to treatment discontinuation and exposure after disease progression.
Median overall survival was 34.9 months, with 12- and 24-month overall survival rates of 85% and 71%, respectively. Durvalumab was discontinued by 133 patients (75%), at a median time of 7.9 months, whereas 27 patients (15%) received at least 12 months of the drug, and 17 (10%) were still undergoing treatment, Dr. Aguiar reported.
Overall, 27% of patients who discontinued durvalumab early received a subsequent regimen, compared with 18% of patients who completed 12 months of treatment with the drug. “The regimen of choice differed according to whether or not the patient had completed the treatment with durvalumab,” he said.
For patients who received durvalumab for up to 12 months, chemotherapy predominated (51%), followed by a tyrosine kinase inhibitor (22%), checkpoint inhibitor plus chemotherapy (20%), and a single-agent checkpoint inhibitor (7%). The five patients who were treated for at least 12 months with durvalumab and then received subsequent therapy were most likely to receive a checkpoint inhibitor plus chemotherapy or a tyrosine kinase inhibitor; one received chemotherapy alone.
The median time to treatment discontinuation with post-durvalumab therapy was 4.2 months. For the 11 patients who received an immune checkpoint inhibitor as a single agent or combined with chemotherapy, the median time to treatment discontinuation (of this subsequent therapy) was 6.2 months, reported Dr. Aguiar.
Study From Taiwan

Chih-Hsi Scott Kuo, MD
The second study was a retrospective analysis of 106 patients with stage III NSCLC, with progression-free survival as the primary endpoint.3 Researchers also evaluated the benefit of durvalumab after chemoradiotherapy consolidation in subgroups based on epidermal growth factor receptor (EGFR) status and expression of PD-L1.
EGFR mutations were confirmed in 11% (higher than the 6% in the PACIFIC trial); 41% of patients had wild-type EGFR, and 48% had unknown EGFR status. PD-L1 expression was at least 1% in 37%, up to 1% in 12%, and unknown in 51%.
Median progression-free survival was 11.8 months; the progression-free survival rate was 49% at 12 months and 44% at 18 months. For most patients (84%), durvalumab was started more than 42 days after radiotherapy. Whether this delay impacted progression-free survival needs further investigation, according to lead investigator Chih-Hsi Scott Kuo, MD, of Linkou Chang Gung Memorial Hospital, Taipei, Taiwan.
The 1-year progression-free survival according to subgroups is shown in Table 1. EGFR status was a factor in the length of remission, but PD-L1 status was not.
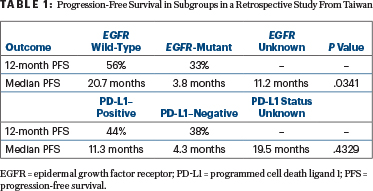
Dr. Kuo noted that the data for subgroup analyses should be interpreted with caution because of the small populations, imbalances in baseline characteristics, and other potential confounders. The most common adverse events were respiratory, including pneumonitis, which occurred in 21% of patients.
Study From Croatia
The overall survival benefit of durvalumab was also confirmed in a single-institution study in Croatia.4 In this study of 42 patients from the University Hospital Centre Zagreb, median overall survival was not reached. Cumulative survival at the end of data cutoff, after a median of 32 months of treatment, was 66.7%, according to Marko Jakopovic, MD.
Altogether, 21 patients (50%) completed the planned 12 months of treatment; 14 (33%) had disease progression during durvalumab therapy, and 7 (17%) discontinued therapy earlier because of adverse events.
Median progression-free survival was 24.1 months, with longer remissions observed in female (median not reached) than in male patients (17.2 months; P = .042). No age-related differences were observed, according to Dr. Jakopovic. Patients with PD-L1 expression of at least 50% had significantly longer progression-free survival than those with lower PD-L1 expression (median 8.1 months vs not reached; P = .0009).
“Surprisingly, disease stage and nodal status did not influence progression-free survival, nor did tumor histology,” Dr. Jakopovic said. “A significant progression-free survival benefit was observed among patients who underwent concurrent chemoradiotherapy, compared with those who were treated sequentially (not reached vs 5.1 months; P = .0008),” he added.

Marko Jakopovic, MD
Durvalumab was initiated at a median time of 45 days from the day of radiation therapy completion. Although a significant number of patients received durvalumab therapy with a long time lag after radiotherapy completion, no differences regarding progression-free survival were observed between those whose immunotherapy was started within the recommended 6-week period compared with others, Dr. Jakopovic reported.
Patients whose side effects caused treatment delay or cessation did not have a shorter progression-free survival. The most common adverse event was pneumonitis, any grade of which occurred in 40% of patients but was grade 3 or 4 in just one patient.
DISCLOSURE: Dr. Aguiar has received honoraria from AstraZeneca, Boehringer Ingelheim, MSD, Aché, Novartis, Amgen, Pfizer, and Bayer. Dr. Kuo has received speaker honoraria from AstraZeneca, Boehringer Ingelheim, Roche, Pfizer, Eli Lilly, Novartis, Ono Pharma, Chugai, Merck, and Guardant Health and has served as a consultant to AstraZeneca Boehringer Ingelheim, Eli Lilly, Chugai, Merck, Takeda, and Novartis. Dr. Jakopovic has received advisory/consultancy and speakers bureau/expert fees from AstraZeneca, Hoffmann–La Roche, Merck Sharp Dohme, Bristol Myers Squibb, Novartis, and Boehringer Ingelheim.
REFERENCES
1. Antonia SJ, Villegas A, Daniel D, et al: Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377:1919-1929, 2017.
2. Aguiar P, de Marchi PM, Montella TC, et al: Real-world evidence of durvalumab for stage III NSCLC: Survival outcomes and patterns of care post-progression. 2022 World Conference on Lung Cancer. Abstract WS07.06. Presented August 6, 2022.
3. Yang TY, Wang CC, Hua CC, et al: First real-world data on unresectable stage III NSCLC patients treated with durvalumab after chemoradiotherapy in Asia area. 2022 World Conference on Lung Cancer. Abstract EP05.01-003. Presented August 6, 2022.
4. Gabelica AB, Seiwerth F, Bitar L, et al: Chemoradiotherapy followed by durvalumab in unresectable advanced NSCLC. 2022 World Conference on Lung Cancer. Abstract EP05.01-018. Presented August 6, 2022.

