Indication
Crizotinib (Xalkori) is an oral inhibitor of receptor tyrosine kinases including anaplastic lymphoma kinase (ALK), hepatocyte growth factor receptor (HGFR, c-Met), and recepteur d’origine nantais (RON). In August 2011, the FDA granted the drug accelerated approval for the treatment of patients with locally advanced or metastatic non–small cell lung cancer (NSCLC) that is positive for rearrangements of the ALK gene as detected by an FDA-approved test. 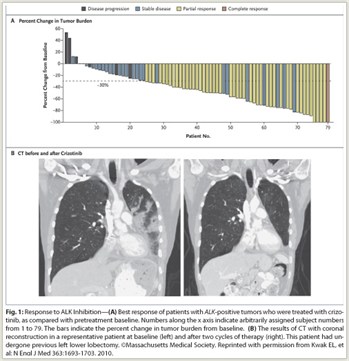 Concurrent with crizotinib approval, the FDA approved the Vysis ALK Break-Apart FISH (fluorescence in situ hybridization) Probe Kit, a companion diagnostic test designed to detect these rearrangements.
Concurrent with crizotinib approval, the FDA approved the Vysis ALK Break-Apart FISH (fluorescence in situ hybridization) Probe Kit, a companion diagnostic test designed to detect these rearrangements.
This indication for crizotinib is based on response rate observed in two single-arm trials in patients with locally advanced or metastatic ALK-positive NSCLC, most of whom had received prior systemic therapy (Fig. 1). No available data show improvement of survival. In one study in 136 patients, which used the Vysis kit to identify ALK-positive NSCLC, there was 1 complete response and 67 partial responses, for an overall response rate of 50%. Median duration of treatment was 22 weeks, and median response duration was 42 weeks. In another study in 119 patients, which used several local clinical trial assays to identify ALK-positive NSCLC, there were 2 complete responses and 69 partial responses, for an overall response rate of 61%. Median duration of treatment was 32 weeks and median response duration was 48 weeks. Responses were observed within 8 weeks in 79% and 55% of responders in the two trials.
How It Works
 Translocations in the ALK gene can result in the expression of oncogenic fusion proteins. The formation of ALK fusion proteins results in activation and dysregulation of the expression and signaling of the gene, which can contribute to increased cell proliferation and survival in tumors expressing these proteins. Crizotinib inhibits ALK and c-Met phosphorylation, blocking activity of the gene; it has shown antitumor activity in xenografts expressing EML4- or NPM-ALK fusion proteins or c-MET. Approximately 5% of NSCLC patients harbor ALK translocations.
Translocations in the ALK gene can result in the expression of oncogenic fusion proteins. The formation of ALK fusion proteins results in activation and dysregulation of the expression and signaling of the gene, which can contribute to increased cell proliferation and survival in tumors expressing these proteins. Crizotinib inhibits ALK and c-Met phosphorylation, blocking activity of the gene; it has shown antitumor activity in xenografts expressing EML4- or NPM-ALK fusion proteins or c-MET. Approximately 5% of NSCLC patients harbor ALK translocations.
How It Is Given
Crizotinib is taken orally twice daily at a dose of 250 mg for as long as the patient is deriving clinical benefit. Capsules are swallowed whole and can be taken with or without food. If required, dose reductions are first to 200 mg twice daily and then to 250 mg once daily.
Safety Profile
 The most common adverse reactions (≥ 25%) observed in both studies supporting crizotinib approval were vision disorder, nausea, diarrhea, vomiting, edema, and constipation. Vision disorders included visual impairment, photopsia, blurred vision, vitreous floaters, photophobia, and diplopia. Grade 3 or 4 adverse reactions in at least 4% of patients included increased ALT and neutropenia. Severe, life-threatening, or fatal treatment-related pneumonitis occurred in 1.6% of crizotinib patients in clinical trials. All cases occurred within 2 months after treatment initiation.
The most common adverse reactions (≥ 25%) observed in both studies supporting crizotinib approval were vision disorder, nausea, diarrhea, vomiting, edema, and constipation. Vision disorders included visual impairment, photopsia, blurred vision, vitreous floaters, photophobia, and diplopia. Grade 3 or 4 adverse reactions in at least 4% of patients included increased ALT and neutropenia. Severe, life-threatening, or fatal treatment-related pneumonitis occurred in 1.6% of crizotinib patients in clinical trials. All cases occurred within 2 months after treatment initiation.
Cost—The cost of crizotinib therapy is estimated at $9,600 per month, or $115,000 per year. ■
Suggested Readings
Cui JJ, Tran-Dube M, Shen H, et al: Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem 54:6342-6363, 2011.
Hallberg B, Palmer RH: Crizotinib—latest champion in the cancer wars? N Engl J Med 363:1760-1762, 2010.
Kwak EL, Bang Y-J, Camidge DR, et al: Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 363:1693-1703, 2010.
U.S. Food and Drug Administration: Crizotinib. Available at http://www.fda.gov/AboutFDA/CentersOffices/CDER/ucm270058.htm. Accessed October 13, 2011.
XALKORI® (crizotinib) capsules, oral. Prescribing information. Pfizer Labs, August 2011. Available at http://labeling.pfizer.com/showlabeling.aspx?id=676. Accessed October 13, 2011.

