Based on multiple phase III prospective trials, there is evidence that both PARP inhibitors and antiangiogenic therapies such as bevacizumab provide benefit when utilized in a maintenance strategy in the first-line treatment of advanced epithelial ovarian cancer (GOG 218, ICON7, SOLO-1, PRIMA, VELIA). The recent publication of PAOLA-1 by Ray-Coquard et al1 in The New England Journal of Medicine was one of three positive phase III trials published in the same issue on December 19, 2019. Each of the three recent trials met the primary endpoint in the intent-to-treat population (all comers) utilizing a PARP inhibitor for maintenance therapy.

David M. O’Malley, MD

Antonio Castaneda, MD
These trials were preceded by the publication of SOLO-1, which showed a marked improvement in progression-free survival with olaparib maintenance in patients with ovarian cancer who had a BRCA mutation (germline or somatic)—leading to the U.S. Food and Drug Administration (FDA) approval of olaparib on December 19, 20182—exactly 1 year prior to the publication of PAOLA-1. The FDA approved bevacizumab for first-line treatment with a platinum-based chemotherapy followed by maintenance on June 13, 2018, based on the positive results of GOG 2183 and further supported by the results of ICON7.4
The benefit of a PARP inhibitor has been most dramatically seen in patients with BRCA1 or BRCA2 mutations. BRCA1 and BRCA2 play a role in homologous recombination repair. Therefore, investigators are actively studying the efficacy of PARP inhibitors in patients who have deficiency in homologous recombination repair (HRD).
Preclinical studies have shown that the inhibition of angiogenesis leads to hypoxic environments within the tumor, potentially leading to a BRCA-like phenotype.5 This supposition contributed to the design of PAOLA-1, a phase III randomized placebo-controlled trial, which looked at the benefit of the addition of the PARP inhibitor olaparib for 24 months to patients with advanced-stage epithelial ovarian cancer receiving cytotoxic chemotherapy with bevacizumab given as maintenance therapy (15 mg/kg every 3 weeks) for up to 15 months.
OF NOTE
On April 29, 2020, the FDA approved niraparib (Zejula) for the maintenance treatment of adult patients with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to first-line platinum-based chemotherapy.Closer Look at PAOLA-1
PAOLA-1 randomly assigned patients with advanced-stage epithelial ovarian cancer who were receiving cytotoxic chemotherapy with a platinum-taxane combination, either as neoadjuvant treatment or
adjuvant treatment, with bevacizumab as maintenance therapy in a 2:1 ratio to receive olaparib maintenance therapy or placebo in addition to bevacizumab. The progression-free survival data published in PAOLA-1, which are summarized in this issue of The ASCO Post, support the conclusion that the addition of olaparib to bevacizumab increases progression-free survival in patients receiving front-line therapy with a platinum-taxane combination compared to bevacizumab alone as maintenance therapy (median = 22.1 vs 16.6 months, hazard ratio [HR] = 0.59, 95% confidence interval [CI] = 0.49–0.72, P < .001) in the intent-to-treat population.
In order to show consistency of the treatment effect in prespecified subgroups, a preplanned progression-free survival analysis was performed in which the hazard ratio and 95% confidence interval were calculated. In patients with mutations in BRCA, there was a benefit to the addition of olaparib to bevacizumab (median = 37.2 vs 21.7 months, HR = 0.31, 95% CI = 0.20–0.47). An HRD score ≥ 42 (according to the myChoice HRD Plus assay) was defined as “positive for HRD,” as compared to those who had homologous recombination–proficient (“HRD-negative”) tumors. Olaparib in combination with bevacizumab showed improved progression-free survival in patients who were HRD-positive without a BRCA mutation (median = 28.1 vs 16.6 months, HR = 0.43, 95% CI = 0.28–0.66) compared to those who were HRD-negative (median = 16.6 vs 16.2 months, HR = 1.00, 95% CI = 0.75–1.35).
PAOLA-1 provides further evidence of the benefit of utilizing a PARP inhibitor in the front-line maintenance setting for patients with advanced-stage epithelial ovarian cancer. One of the most important aspects of the design of PAOLA-1 is the control arm of the trial, which received active maintenance with bevacizumab, whereas in each of the other recently positive phase III trials utilizing a PARP inhibitor strategy, the control arm was placebo (observation).
Patients with mutations in BRCA1 or BRCA2 have consistently shown a benefit from the addition of a PARP inhibitor as maintenance therapy, in PAOLA-1 and other studies (SOLO-1,2 PRIMA,6 VELIA7). Unfortunately, there was not a third arm in the PAOLA-1 study evaluating olaparib alone. This leaves us to question whether a PARP inhibitor combined with bevacizumab would be superior to a PARP inhibitor alone as maintenance therapy in the intent-to-treat population. Several ongoing or recently completed studies are trying to answer this question (see Table 1).
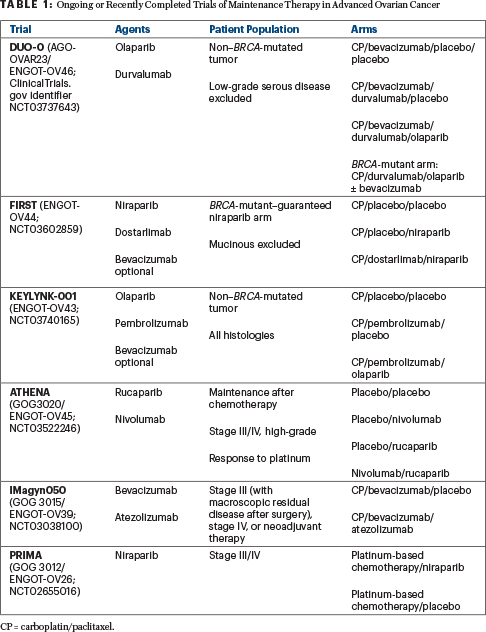
This is even more important when we look at the patients with BRCA-mutated tumors. It is important to note that cross-trial comparisons are flawed in terms of analytic interpretations and are not valid comparisons. In SOLO-1, the inclusion criteria were similar to PAOLA-1, except all tumors in SOLO-1 had a BRCA mutation and patients did not receive bevacizumab with their first-line chemotherapy. In SOLO-1,1 progression-free survival at 3 years was 60%, whereas in PAOLA-1 it was similar though slightly higher (approaching 70%). Without a comparator arm of olaparib maintenance alone, it is not possible to definitely state that the olaparib combination with bevacizumab is a better option than single-agent olaparib in patients with BRCA-mutated tumors, though the slightly higher progression-free survival at 3 years is intriguing.
Other Subgroup Considerations
In a prespecified subgroup analysis of PAOLA-1, one of the most interesting findings was in patients who were HRD-positive without a somatic BRCA mutation. In these patients, there was a doubling of the 24-month progression-free survival (52% vs 26%), with the difference being statistically significant (HR = 0.43, 95% CI = 0.28–0.66). This group of patients in the prespecified subgroup analysis appeared to derive the most benefit from the combination of olaparib and bevacizumab. It is not clear whether these results are being driven by the combination or if olaparib alone provided the marked improvement in the outcomes.
In PRIMA,6 the myChoice HRD Plus assay was used with the same cutoff score of 42. In patients with tumors found to be HRD and without a BRCA mutation, the magnitude of benefit with niraparib was similar to that with the combination of olaparib/bevacizumab (HR = 0.5, 95% CI = 0.31–0.83). Of course, the benefit in PAOLA-1 was compared to the active bevacizumab arm, not a placebo. Additionally, it is not clear based on how the myChoice HRD Plus assay is reported if a group of patients with tumors with non-BRCA homologous recombination repair mutations (eg, RAD51C, RAD51D) are driving the benefits. The results of PAOLA-1 in the HRD-positive group of patients are impressive, but one must consider how much of the benefit is being driven by olaparib alone.
In PAOLA-1, HRD-negative tumors were predicted, based on preclinical studies, to respond similar to a BRCA-like phenotype when treated with antiangiogenic therapeutics. It was hypothesized that the addition of a PARP inhibitor to bevacizumab compared to bevacizumab alone would be beneficial. In the prespecified subgroup analysis of HRD-negative tumors, there was no improvement with the combination (median progression-free survival = 16.6 vs 16.2 months, HR = 1.00, 95% CI = 0.75–1.35). The progression-free survival is very similar to that seen in GOG 218 (14.1 months) in the bevacizumab maintenance arm.3 It is very important to consider that PAOLA-1 was not powered to show statistically significant differences in these prespecified subgroup analyses.
PRIMA showed a benefit with niraparib maintenance therapy compared to placebo (median progression-free survival = 8.1 vs 5.4 months, HR = 0.68, 95% CI = 0.49–0.94) in the patients with tumors that were HRD-negative.6 The best maintenance strategy in patients with HRD-negative tumors is unclear. The maintenance options include treating patients with a PARP inhibitor or bevacizumab, but it does not appear that the combination of a PARP inhibitor and bevacizumab is the best option in homologous recombination–proficient tumors (HRD-negative). It follows that the greatest unmet medical need in the setting of newly diagnosed ovarian cancer is among patients who are homologous recombination–proficient (or otherwise “biomarker-negative”).
Adverse Events
The addition of olaparib to bevacizumab did not greatly increase the rate of grade 3 adverse events (57% vs 51%). There was an expected increase in the rate of anemia (17% vs 1%) and fatigue (5% vs 1%) with this combination. The development of myelodysplastic syndromes, acute myeloid leukemia, or aplastic anemia in the PAOLA-1 trial was similar (1% in the combination arm vs < 1% in the bevacizumab-alone arm) to that reported in other clinical trials.
Of particular note, there was a decreased rate of all-grade and grade ≥ 3 hypertension in patients receiving olaparib with bevacizumab compared to bevacizumab alone. However, it is unclear whether the interaction of PARP inhibition with the vascular endothelial growth factor inhibitor could contribute to the lower levels of hypertension seen in the combination arm of PAOLA-1.
“We should no longer be questioning whether maintenance therapy is the best option [in the first-line setting of advanced ovarian cancer]; it is time to ask which maintenance strategy is optimal.”— David M. O’Malley, MD, and Antonio Castaneda, MD
Tweet this quote
Conclusions
PAOLA-1 demonstrated a benefit of the addition of olaparib to bevacizumab as maintenance therapy after platinum-taxane combination therapy for advanced-stage epithelial ovarian cancer in the intent-to-treat populations. In a prespecified subgroup analysis, patients with tumors with a BRCA mutation and patients with homologous recombination–repair deficiency (non–BRCA-mutated) clearly benefited from the combination of olaparib and bevacizumab. In HRD-negative tumors, it is unclear if one can conclude whether the addition of olaparib to bevacizumab maintenance therapy is beneficial. The toxicity profile was as expected, with an increased incidence of anemia and fatigue. We now have multiple, randomized, phase III studies consistently demonstrating a progression-free survival benefit with the addition of maintenance therapy to front-line carboplatin and paclitaxel with a PARP inhibitor, an antiangiogenic therapeutic agent (bevacizumab), or both.
It is necessary to counsel all patients with first-line advanced ovarian cancer on the clear benefit of maintenance therapy. We should no longer be questioning whether maintenance therapy is the best option; it is time to ask which maintenance strategy is optimal. All patients diagnosed with ovarian cancer should be encouraged to participate in a clinical trial, so we can determine the best maintenance option for each individual patient based on clinical factors (such as stage IV disease, suboptimal debulking procedures, or the need for neoadjuvant chemotherapy), biomarker status (eg, BRCA, HRD, or homologous recombination repair mutations), or other subgroups yet to be defined. Ongoing studies (see Table 1) are attempting to answer these questions as well as to identify the role of immune-oncology agents in the treatment of women with advanced ovarian cancer.
Dr. O’Malley is Director of the Division of Gynecologic Oncology, and Dr. Castaneda is a gynecologic oncology fellow at the Ohio State University Medical Center, Columbus.
DISCLOSURE: Dr. O’Malley has served in a consulting or advisory role for AbbVie, Agenus, Ambry Genetics, AstraZeneca, Clovis Oncology, Eisai, Genelux, Genentech/Roche, GOG Foundation, Immunogen, Iovance Biotherapeutics, Janssen Oncology, Leap Therapeutics, Marker Therapeutics, Novocure, OncoQuest, Tarveda Therapeutics, Tesaro, Translational Genomics/Cordgenics; and has received institutional research funding from AbbVie, AbbVie/Stemcentrx, Agenus, Ajinomoto, Amgen, Array BioPharma, AstraZeneca, Bristol-Myers Squibb, Cerulean Pharma, Clovis Oncology, EMD Serono, Ergomed, Genentech/Roche, Genmab, Immunogen, Iovance Biotherapeutics, Janssen Research & Development, Leap Therapeutics, Merck, PharmaMar, Regeneron, Seattle Genetics, Tesaro, and Tracon Pharma. Dr. Castaneda reported no conflicts of interest.
REFERENCES
1. Ray‑Coquard I, Pautier P, Pignata S, et al: Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381:2416-2428, 2019.
2. Moore K, Colombo N, Scambia G, et al: Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379: 2495-2505, 2018.
3. Burger RA, Brady MF, Bookman MA, et al: Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473-2483, 2011.
4. Oza AM, Cook AD, Pfisterer J, et al: Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase III randomised trial. Lancet Oncol 16:928-936, 2015.
5. Fanale D, Bazan V, Caruso S, et al: Hypoxia and human genome stability: Downregulation of BRCA2 expression in the breast cancer cell line. Biomed Res Int. September 22, 2013 (early release online).
6. González-Martín A, Pothuri B, Vergote I, et al: Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381: 2391-2402, 2019.
7. Coleman RL, Fleming GF, Brady MF, et al: Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med 381:2403-2415, 2019.

