
Syed Ali Abutalib, MD

Vaishali Sanchorawala, MD
Systemic amyloid light-chain (AL) amyloidosis is characterized by the deposition of immunoglobulin light chains, produced by clonal CD38-positive plasma cells, as insoluble amyloid fibrils in vital organs. It is a disease that can progress rapidly and is fatal without treatment. The past decade has seen remarkable advancements that have instilled hope among patients with this disease.1
Daratumumab-Based Regimen
ABSTRACT 891 (ASH 2024): Subcutaneous daratumumab plus bortezomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed AL amyloidosis: ANDROMEDA study final analysis (ClinicalTrials.gov identifier NCT03201965)2,3
Background: ANDROMEDA is a randomized, open-label, active-controlled phase III trial of bortezomib, cyclophosphamide, and dexamethasone (VCd) ± daratumumab in patients with newly diagnosed AL amyloidosis. Daratumumab plus VCd is the first and only approved regimen for this condition and is considered a standard of care for patients with newly diagnosed AL amyloidosis.
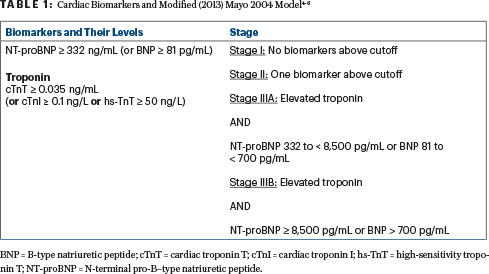
Methods: Key eligibility criteria included newly diagnosed AL amyloidosis with measurable hematologic disease, at least one involved organ, cardiac stage (Mayo 20044,5; Table 14-6) I to IIIA (excluded patients with Mayo 2004 stage IIIB), estimated glomerular filtration rate ≥ 20 mL/min, and absence of symptomatic multiple myeloma. Patients were randomly assigned 1:1 (n = 338) to receive VCd ± daratumumab and thereafter daratumumab every 4 weeks for up to 24 cycles (28-day cycles). The primary endpoint was the overall hematologic complete response rate (defined asthe absence of symptom-related changes). Secondary endpoints included major organ deterioration progression-free survival (ie, the absence of end-stage renal or cardiac disease, hematologic disease progression, or death), overall survival, organ response rate, and safety.
Results: Investigators reported the results of the preplanned final analysis for freedom from major organ deterioration progression-free survival and overall survival. Of the 122 patients given VCd who received subsequent therapy, 82 patients (67%) received subsequent daratumumab. Median follow-up was 61.4 months (range, 0.0–71.2 months).
The hematologic complete response rate was 59.5% with daratumumab plus VCd and 19.2% with VCd alone (odds ratio = 6.03 [95% confidence interval (CI) = 3.80–9.58; P < .0001]).
“Despite the small number of patients treated, a slow-go approach may be reasonable for patients with severe advanced amyloid cardiomyopathy....”— SYED ALI ABUTALIB, MD, AND VAISHALI SANCHORAWALA, MD
Tweet this quote
Significant improvements in organ function and overall survival were observed with daratumumab plus VCd vs VCd alone (hazard ratio [HR] for major organ deterioration progression-free survival = 0.44 [95% CI = 0.31–0.63; P < .0001]; HR for overall survival = 0.62 [95% CI = 0.42–0.90; P = .0121]).
Treatment was discontinued for treatment-emergent adverse events in 5% of patients given daratumumab plus VCd and in 4% of those given VCd alone.
Clinical Implications: The addition of subcutaneous daratumumab to VCd resulted in superior outcomes compared with VCd alone, demonstrating deeper and more rapid hematologic responses resulting in a clinically meaningful and statistically significant improvement in both overall survival and major organ deterioration progression-free survival, combined with a 40.7% cardiac complete response. Patient-reported outcomes and quality of life were not found to be compromised by the addition of daratumumab.7
Slow-Go Treatment Approach
ABSTRACT 4672 (ASH 2024): Slow-go treatment in newly diagnosed AL amyloidosis with severe cardiomyopathy.8
Background: Patients with AL amyloidosis are excluded from clinical trials related to elevated cardiac biomarkers, poor cardiac or renal function, or poor performance status. This protocol was created for frail patients whose survival would paradoxically be shortened by full-dose aggressive treatment and who might benefit from a slow-go approach. There are few known therapeutic options for AL amyloidosis with severe cardiac involvement, often defined as 2004 Mayo stage IIIB with NT-proBNP (N-terminal pro-B–type natriuretic peptide) ≥ 8,500 pg/mL and high-sensitivity cardiac troponin T (hs-cTnT) > 54 pg/mL.4,5,9 (See the sidebar for a glossary of terms and more on these staging systems.) A recent European trial (EMN22) of daratumumab monotherapy demonstrated a 50% very good partial hematologic response rate.10 It is important to emphasize that until fibril-targeted treatments become available, plasma cell–directed therapy appears futile in newly diagnosed patients with end-stage cardiomyopathy requiring inotropes or those with a poor performance status.
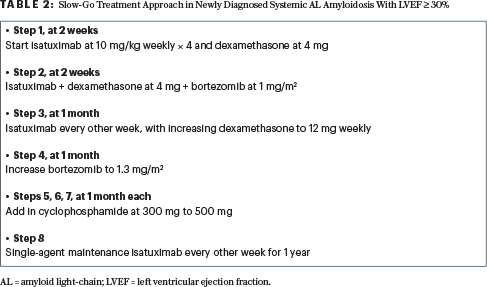
Methods: The primary endpoint was the event-free proportion at 3 months; investigators hypothesized that this would be 40% at 3 months. Inclusion criteria were high-risk, biopsy-proven AL amyloidosis based on NT-proBNP > 8,500 ng/L; hs-cTnT ≥ 50 ng/L4,9; Boston University 2019 stage IIIB criteria [troponin I > 0.1 ng/mL and BNP > 700 pg/mL]11; or Mayo 2012 stage 4 criteria [cTnT≥ 0.025 ng/mL or hs-cTnT ≥ 40 ng/mL plus NT-proBNP ≥ 1,800 pg/mL or BNP ≥ 800 pg/mL plus dFLC (difference between involved and uninvolved free light chains) ≥ 180 mg/L].12 Renal function estimated glomerular filtration rate ≥ 20 mL/min and left ventricular ejection fraction ≥ 30% were required. Exclusion criteria included at least one prior line of therapy, refractory disease to any proteasome inhibitor, or prior anti-CD38 antibody exposure. Patients were treated in a flexible stepwise approach, as shown in Table 2.
Results: Of 11 patients enrolled, the first 2 patients were on intravenous inotropes at the time of consent, and the eligibility was changed to exclude these patients because of a low likelihood of treatment benefit. In the remainder, three patients died between consent and treatment initiation, two patients died within 30 days of starting treatment, and two patients died between 30 and 90 days of starting treatment. The remaining four patients are in complete hematologic remission; no patients experienced disease progression while on treatment.
Clinical Implications: This slow-go approach may result in a more gradual reduction in light chains than what was reported with daratumumab plus VCd2 without known clinical consequence. Even among patients achieving a hematologic complete response, one moved on to heart transplant, and two others had an improvement in symptomatic heart function. Despite the small number of patients treated, a slow-go approach may be reasonable for patients with severe advanced amyloid cardiomyopathy, especially if they survive the first 3 months of treatment.
DISCLOSURE: Dr. Abutalib reported a financial relationship with AstraZeneca. Dr. Sanchorawala has received research support from Celgene, Millennium-Takeda, Janssen, Prothena, Sorrento, Karyopharm Therapeutics, Oncopeptides, Caelum Biosciences, and Alexion Pharmaceuticals; has served as a consultant for Pfizer, Janssen, Attralus, GateBio, AbbVie, and BridgeBio; and has served on an advisory board for Proclara Biosciences, Caelum Biosciences, AbbVie, Janssen, Regeneron, Protego Biopharma, PharmaTrace, Telix Pharmaceuticals, Prothena Biosciences, AstraZeneca, Nexcella, Alexion Pharmaceuticals, and GlaxoSmithKline.
REFERENCES
1. Sanchorawala V: Systemic light chain amyloidosis. N Engl J Med 390:2295-2307, 2024.
2. Kastritis E, Palladini G, Minnema MC, et al: Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med 385:46-58, 2021.
3. Kastritis E, Palladini GO, Minnema MC, et al: Subcutaneous daratumumab + bortezomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed light chain amyloidosis: Overall survival and final major organ deterioration progression-free survival results from the phase 3 ANDROMEDA study. 2024 ASH Annual Meeting & Exposition. Abstract 891. Presented December 9, 2024.
4. Dispenzieri A, Gertz MA, Kyle RA, et al: Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: A staging system for primary systemic amyloidosis. J Clin Oncol 22:3751-3757, 2004.
5. Wechalekar AD, Schonland SO, Kastritis E, et al: A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood 121:3420-3427, 2013.
6. Palladini G, Milani P, Merlini G: Predicting survival in light chain amyloidosis. Haematologica 104:1294-1296, 2019.
7. Sanchorawala V, Palladini G, Minnema MC, et al: Health-related quality of life in patients with light chain amyloidosis treated with bortezomib, cyclophosphamide, and dexamethasone ± daratumumab: Results from the ANDROMEDA study. Am J Hematol 97:719-730, 2022.
8. Hofmeister CC, Gupta VA, Joseph NS, et al: A slow-go treatment in newly diagnosed AL amyloidosis with severe cardiomyopathy. 2024 ASH Annual Meeting & Exposition. Abstract 4672. Presented December 9, 2024.
9. Muchtar E, Kumar SK, Gertz MA, et al: Staging systems use for risk stratification of systemic amyloidosis in the era of high-sensitivity troponin T assay. Blood 133:763-766, 2019.
10. Kastritis E, Minnema MC, Dimopoulos MA, et al: Efficacy and safety of daratumumab monotherapy in newly diagnosed patients with stage 3B light-chain amyloidosis: A phase 2 study by the European Myeloma. Blood 142(suppl 1):539, 2023.
11. Lilleness B, Ruberg FL, Mussinelli R, et al: Development and validation of a survival staging system incorporating BNP in patients with light chain amyloidosis. Blood 133:215-223, 2019.
12. Kumar S, Dispenzieri A, Lacy MQ, et al: Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol 30:989-995, 2012.
Dr. Abutalib is Director of the Malignant Hematology and Transplantation & Cellular Therapy Programs at the Advocate/Aurora St. Luke’s Medical Center, Milwaukee, and Associate Professor at Rosalind Franklin University of Medicine and Science, Chicago. Dr. Sanchorawala is Professor of Medicine and Director, Amyloidosis Center, Boston Medical Center and Boston University Chobanian & Avedisian School of Medicine, Boston.
UNDERSTANDING AL AMYLOIDOSIS
Glossary of Terms
BNP: Brain natriuretic peptide
cTnI: Cardiac troponin I
cTnT: Cardiac troponin T
dFLC: Difference between involved and uninvolved serum free light chains
hs-TnT: High-sensitivity troponin T
NT-proBNP: N-terminal pro-B-type natriuretic peptide
Staging Systems
Mayo 2012 Staging System: A way to classify patients based on the severity of cardiac involvement, using cardiac biomarkers such as troponin (Tn ≥ 0.025 µg/L and NT-proBNP ≥ 1,800 ng/L plus dFLC ≥ 18 mg/dL as risk factors. If cTnT is measured using a high-sensitivity assay (hs-TnT), a cutoff of 40 ng/L should be used. It helps to predict prognosis and guide treatment decisions, particularly in relation to cardiac involvement.
- Stage I: Low risk based on normal or low levels of cardiac biomarkers (hs-TnT, NT-proBNP) and dFLC
- Stage II: Intermediate risk, with elevated levels of at least one biomarker
- Stage III: Higher risk, with elevated levels of multiple biomarkers
- Stage IV: Highest risk, with elevated three biomarkers damage
Boston University (BU) Staging System: Published in 2019, it uses two biomarkers to stratify patients based on levels of BNP and cTnI to assess the severity of cardiac disease and predict survival in patients with this condition. It is similar to the Mayo 2004 staging system (except the BU system uses BNP and cTnI instead of NT-proBNP and TnT).
- Stage I: Neither BNP > 81 pg/mL nor TnI > 0.1 ng/mL
- Stage II: Either BNP > 81 pg/mL or TnI > 0.1 ng/mL (but not both)
- Stage IIIA: Both BNP > 81 pg/mL and TnI > 0.1 ng/mL
- Stage IIIB: Modified version of stage IIIA, which uses a higher BNP cutoff (700 pg/mL)

