
Steven E. Vogl, MD
At the 2019 ASCO Annual Meeting, and simultaneously in The New England Journal of Medicine, we heard the third paper reporting results from TAILORx.1,2 The first, in 2015,3 indicated that women with node-negative breast cancers with Oncotype DX recurrence scores less than 11 did extremely well with just hormonal therapy (9-year distant recurrence rate later shown to be 3.2%). In the second, presented and published in 2018,4 we learned that chemotherapy added nothing, or next to nothing, to reducing the 9-year rate of distant recurrence for women with node-negative breast cancers and Oncotype DX recurrence scores of 11 to 25. This was a major contribution and spares the vast majority of women who have hormone receptor–positive, HER2-negative breast cancers with uninvolved nodes the unpleasantness and toxicity of chemotherapy.
The first objective of the current paper was to look at whether the usual clinical information we collect is still relevant to the prognosis of breast cancer in an era in which prognosis is predominantly decided by gene-expression profiles. The answer to this question is a resounding yes. The authors of this paper chose a simple binary system of clinical classification that had been previously used in the MINDACT trial.5 This system was based on largest tumor diameter and histologic grade assigned according to microscopic examination of the cancer tissue. To be called “low risk,” a high-grade tumor had to be 1 cm or less in largest diameter, an intermediate-grade tumor had to be 2 cm or less in diameter, and a low-grade tumor had to be 3 cm or less in diameter. Any tumor not qualifying as “low risk” was considered “high risk.”
This simple system added substantially to the use of recurrence score in predicting the 9-year incidence of distant recurrence across all ages and recurrence scores and did so whether or not the patient received chemotherapy in addition to endocrine therapy. For 6,496 women with intermediate recurrence scores of 11 to 25, having a clinical risk that was “high” increased the risk of distant metastases 2.42-fold. This information is very important in defining a risk level below which no further intervention is needed. Because the TAILORx study cohort is so large, we can use its results to confidently assign excellent prognoses to many women with node-negative, hormone receptor–positive, HER2-negative breast cancers.
Tumor diameter and histologic grade are also important in categorizing these women into groups to define the absolute benefit of an added intervention—the greater the risk, the greater the room for substantial benefit. The TAILORx investigators did both in the 2019 paper, and we will review the results here.
“TAILORx will likely define the standards of breast cancer care for parts of the world in which the Oncotype DX test is widely used.”— Steven E. Vogl, MD
Tweet this quote
We now know that to assess the prognosis, we need not only a gene-expression profile, but also a ruler to measure the largest diameter of the cancer and a skilled pathologist with a microscope and the knowledge to use it to assign a histologic grade to the tumor. When the Oncotype DX test was new, the first big confirmation studies applied it using stored specimens from already completed NSABP studies. Dr. Norman Wolmark, then NSABP Chairman, showed a slide in which a microscope was surrounded by a circle and obscured by a diagonal line that covered the microscope. The microscope, he said, was an obsolete instrument we no longer needed. According to the 2019 TAILORx analysis, this is not the case. We now need histologic grade in addition to recurrence score and tumor size to best estimate the prognosis.
TAILORx will likely define the standards of breast cancer care for parts of the world in which the Oncotype DX test is widely used. That said, the analysis did not strictly show that one needs histologic grade to define prognosis—only that, when using tumor size and grade, definition of prognosis was improved. The analytic exercise of ranking and quantitating contributions to prognosis in a multivariate analysis is straightforward and has not yet been presented for TAILORx.
Further, the TAILORx study chairman, Dr. Joseph Sparano, has noted that important information may have been hidden by dividing tumor size into four groups and histologic grade into three groups, and then dividing the resulting 12 categories into low and high risk. The size cutpoints are obviously arbitrary—is a 1.1-cm high-grade tumor really cursed with a much worse prognosis than a 1.0-cm tumor? Of course not. The grade cutpoints are more subtle, since three histologic characteristics are each scored on a scale of 1 to 3, then added to create a total “Nottingham” score of 3 to 9, which is then divided into low-, intermediate-, or high-grade categories.
Dr. Sparano promises to later present analyses of prognosis using size and grade as continuous variables. When he does so, we need to remember that we will need confirmation of the validity of the scoring system in an independent data set before we can confidently apply it.
Size and Grade Did Not Predict Chemotherapy Benefit
Although clinical risk is important in defining prognosis, it did not affect the lack of benefit from the addition of chemotherapy to hormonal therapy among women whose cancers had intermediate recurrence scores between 11 and 25. There was a subtle possible exception that leads to the most interesting hypothesis generated from TAILORx in the 2019 analysis: that chemotherapy reduces the risk of distant metastases at higher, but still intermediate, recurrence scores among women under age 50 years when it irreversibly destroys ovarian function, but not in situations in which ovarian function recovers. Further, assessed clinical risk modifies the magnitude of the absolute benefit.
What About Young Women With High-Intermediate Recurrence Scores?
In the 2018 paper, Dr. Sparano and his coauthors noted that there seems to be an exception to the demonstrated lack of benefit to adding chemotherapy to hormonal therapy for women whose node-negative breast cancers had intermediate recurrence scores. This exception involved identifying a subgroup in which chemotherapy worked, when the primary analysis showed that it did not.
That subgroup consisted of women less than 50 years old with recurrence scores of 16 to 25. A similar subgroup defined by using premenopausal status rather than age apparently did not benefit from chemotherapy. As pointed out in a column in these pages in 2018,6 the procedure used to identify this subgroup is statistically suspect and should be considered “hypothesis-generating,” rather than definitive evidence. Also, it is philosophically displeasing to have a large, well-done study demonstrating that chemotherapy adds nothing, and then assert, “Well, chemotherapy adds benefit for some small group.”
Dr. Sparano has argued, however, that finding a subset that benefits in a negative superiority trial is not the same as finding a similar subset in a noninferiority trial such as TAILORx. Moreover, he maintained, it was important to ensure that there was no subgroup of patients who could be deriving some benefit but were lumped together with those who clearly had no benefit. I, in turn, argue that such groups will always be discoverable depending on the play of chance and the creativity of the analysts. I argue for extreme caution in using such subgroup analyses in patient care, since the number of possible subgroups is infinite.
“Although clinical risk is important in defining prognosis, it did not affect the lack of benefit from the addition of chemotherapy to hormonal therapy among women whose cancers had intermediate recurrence scores between 11 and 25.”— Steven E. Vogl, MD
Tweet this quote
In the 2019 paper, a hypothesis is offered that reconciles the major finding of the lack of benefit of chemotherapy for women with recurrence scores of 11 to 25 and the apparent benefit of chemotherapy in younger women with recurrence scores of 16 to 25. This hypothesis reconciles this paradox with Talmudic elegance. The core of the reconciliation hypothesis is that, for the women who were premenopausal in their 40s at entry, chemotherapy functioned as an intensification of hormonal therapy by ablating ovarian function. This means that the chemotherapy was producing benefit not as chemotherapy, but as ovarian ablation. It leaves the essential conclusion of the 2018 TAILORx paper intact, with an asterisk explaining that intensification of hormonal therapy with ovarian ablation appears to improve results in premenopausal women aged 40 to 50 years with high-intermediate recurrence scores.
The evidence suggests this hypothesis: benefit from chemotherapy among women under age 50 is restricted to women over 40, and especially those over 45, whose ovaries are likely to permanently fail when exposed to chemotherapy including cyclophosphamide. Among women under 40, whose ovarian function is likely to resume after chemotherapy, there was no apparent benefit from chemotherapy. These results are summarized in Table 1, using the most relevant endpoint for adjuvant therapy of good-prognosis breast cancer—freedom from distant recurrence after a given period of observation (termed “distant recurrence–free interval” by the STEEP consortium.7
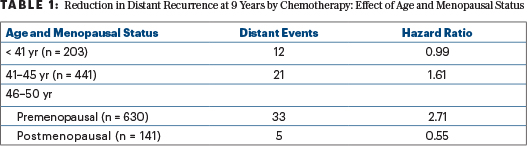
Note that this hypothesis that “chemotherapy benefit is via ovarian ablation” depends on two small groups with recurrence scores of 16 to 25 and very few events in each—12 distant events among 203 women aged ≤ 40 who had chemotherapy or observation and no chemotherapy benefit, and 5 distant events among 141 postmenopausal women aged 46 to 50 who had chemotherapy or observation and fewer of the distant events in the observation group. A shift of one or two events in either group would change the results.
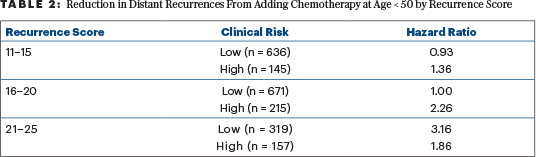
The hypothesis is further complicated when clinical risk (high or low) is added to the analysis (Table 2). The addition of chemotherapy has no apparent benefit in reducing 9-year distant recurrence among 671 women aged ≤ 50 with low clinical risk and recurrence scores of 16 to 20. These women account for 49% of all the women aged ≤ 50 with recurrence scores of 16 to 25.
Chemotherapy did have apparent benefit for those with recurrence scores of 16 to 20 with high clinical risk and among all women with recurrence scores of 21 to 25. Together, 691 such women were assigned to chemotherapy or observation in TAILORx. This complicated sorting generates a high likelihood of random apparent associations that may not be reproducible. The authors of the 2019 paper acknowledged this and made no effort to correct their analyses for multiple comparisons and the generation of hypotheses after the data were reviewed. They correctly noted that the absolute benefit of adding chemotherapy seems a reasonable result of the interaction of underlying risk and relative risk reduction by adding chemotherapy.
What Do We Do Now?
We need to remember that TAILORx was designed at a time in which adjuvant therapy of breast cancer in North America was largely limited to single-drug hormonal therapy, chemotherapy, or both. Only 13% of premenopausal women with intermediate recurrence scores in TAILORx were subjected to ovarian function suppression. Outcomes according to the use of this modality have not yet been reported. It is possible that chemotherapy failed to benefit women under age 40 with high-intermediate recurrence scores because some of these were women whose ovarian function was already suppressed by goserelin or a similar drug.
If we want to simply apply TAILORx without extrapolation, but still based on subgroup analyses, we can offer chemotherapy similar to that used in TAILORx (largely docetaxel with concurrent cyclophosphamide) to women at “high clinical risk” with recurrence scores of 16 to 20 and to all women with recurrence scores of 21 to 25. The apparent benefits of doing so are outlined in Table 3. Based on the small number of relevant distant events, I would not favor exceptions for women aged < 40 years or those aged 46 to 50 past their menopause. (Their numbers and distant event rates are detailed in Table 1.) The absolute reductions in the 9-year rate of distant metastases of 6% to 9% are enough to justify chemotherapy to most physicians and patients.
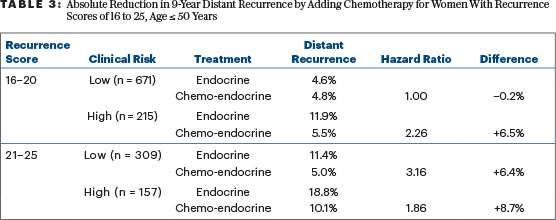
In 2018, I wrote in these pages that the benefits of chemotherapy in patients with recurrence scores of 16 to 20 aged ≤ 50 (an absolute 1.6% reduction in distant metastases at 9 years) were too small to bother with.8 My recommendation changes now: in the 24% of these women with “high clinical risk,” the absolute benefit is 6.5% and worthy of consideration. For those with “low clinical risk” and recurrence scores of 16 to 20 aged ≤ 50 years, no benefit for chemotherapy was demonstrated at all.
Which Chemotherapy Regimen?
Physicians and patients < 50 years old with a recurrence score of 16 to 25 may choose chemotherapy (given the benefits noted in the TAILORx data) rather than ovarian suppression, which was not part of the TAILORx study, except incidentally. In this case, I would still favor chemotherapy that produces less long-term severe toxicity (ie, acute leukemia, cardiac failure, and peripheral neuropathy). In my office, this chemotherapy program is called CMF (cyclophosphamide, methotrexate, and fluorouracil). Because it employs more cyclophosphamide given for more months, CMF is also more toxic to the ovaries than other regimens used in TAILORx. If the “chemotherapy works via ovarian suppression” hypothesis is correct, the added cyclophosphamide should be particularly advantageous for the patient in preventing distant metastases and death from breast cancer.
Offering chemotherapy in violation of the failure of chemotherapy benefit in the entire population with recurrence scores of 11 to 25 based on a subgroup analysis is somewhat “shaky.” It is not much shakier to accept the 2019 “ovarian ablation” hypothesis based on the TAILORx data and use this to offer our patients intensified hormonal therapy.
Who Should Get Ovarian Suppression?
In 2017, I wrote in these pages that it was unclear how to apply data from the SOFT trial on ovarian function suppression in an era when therapy is guided by gene-expression profiles.9 TAILORx begins to help us with this, even though this study did not set out to employ or examine ovarian function suppression. In SOFT, ovarian function suppression most benefited those women who clearly needed chemotherapy. The current TAILORx analysis suggests that this would be any premenopausal woman with a recurrence score > 20, or a premenopausal woman with a recurrence score of 16 to 20 and “high clinical risk.”
We should consider why ovarian function suppression should be useful in women with intermediate recurrence scores. It is likely a result of how the recurrence score was designed and the interplay of risk and sensitivity to hormonal therapy. The recurrence score was designed to predict the prognosis of patients with breast cancer who had no involved axillary nodes and were given 5 years of tamoxifen. Very low recurrence scores (< 11) indicate either a very good prognosis with no systemic therapy or a major benefit from tamoxifen for 5 years. Very high recurrence scores (> 30 in the original analysis) are associated with a worse prognosis without chemotherapy and considerable tamoxifen resistance. In between, benefits from enhanced hormonal therapy (ovarian function suppression, in this case) depend on the interaction of baseline risk (including “clinical risk”) and hormonal sensitivity.
In the “low clinical risk” group with recurrence scores of 16 to 20, both the low risk and sensitivity to tamoxifen alone produce a net of no added benefit for chemotherapy that suppresses ovarian function. With “higher clinical risk” and/or less sensitivity to tamoxifen, chemotherapy that ablates ovarian function can produce substantial benefits (Table 3).
Thus, we should offer ovarian function suppression to clinically high-risk women with a recurrence score of 16 to 20 and to all women with a recurrence score over 20. If the hypothesis is correct, and chemotherapy is working in this population by ablating ovarian function, we can achieve the same ovarian function suppression with less toxicity with either an luteinizing hormone-releasing hormone (LHRH) antagonist or bilateral ovarian resection. Based on SOFT, we know that ovarian suppression is effective at preventing distant metastases and improving overall survival. The current analysis of TAILORx provides risk data to allow us to choose women with sufficient risk of distant metastases to justify suppressing or removing their ovaries.
Benefits of Ovarian Suppression Plus Tamoxifen Based on TAILORx
The benefit of chemotherapy among premenopausal women aged 46 to 50 with recurrence scores of 16 to 25 reduces distant metastases with a hazard ratio of 2.71 favoring chemoendocrine therapy. The 9-year absolute rate of distant metastases falls from 10.0% without chemotherapy to 3.6% with chemotherapy, an absolute benefit of 6.4%. This is consistent with a 5.7% improvement in 8-year disease-free survival from the addition of ovarian suppression to tamoxifen alone seen in SOFT.10 I estimated that distant metastases represented only two-thirds of these events in SOFT.11
If ovarian ablation is how chemotherapy works in these women, ovarian suppression should work as well in women younger than 46 or 41 years with similar, relatively high risks of distant metastases, even if chemotherapy did not work as well for such women in TAILORx. Ovarian function suppression may work better in real life because it works directly, or it may be worse because it requires continued compliance with periodic injections if these are used to reversibly suppress ovarian function, rather than surgical oopherectomy.
Deriving cutpoints for the use of ovarian function suppression from a chemotherapy trial is a questionable extrapolation. It would be preferable to have cutoffs derived from a study that randomly assigned patients to ovarian suppression or not. SOFT is such a trial. Although gene-expression profiling was not mandated in SOFT, the investigators have collected an extensive tissue bank with specimens suitable for Oncotype DX testing. It would be very helpful if Genomic Health would provide Oncotype DX testing for suitable specimens from women randomized to receive either tamoxifen alone or tamoxifen plus ovarian function suppression in SOFT. The International Breast Cancer Study Group, which coordinated SOFT, is willing to provide the specimens. For a comparison to TAILORx, the suitable patients would have to have had no nodal involvement at diagnosis, so only a fraction of the 1,000 women in each arm would need testing for recurrence scores.
Ovarian Suppression Is Only One Way to Intensify Hormonal Therapy
If it turns out that cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors or everolimus reduce the rate of distant metastases among women with hormone receptor–positive breast cancer, then these agents may be used to intensify hormonal therapy either instead of, or in addition to, ovarian function suppression. Studies looking at this strategy are in progress. Inhibitors of PI3 kinase might also intensify hormonal effects in cancers bearing activating mutations in these enzymes, but they are new and no adjuvant studies with these agents have yet been designed, much less launched.
Fulvestrant, expensive and unpleasant injection though it is, is an attractive candidate for intensification of endocrine therapy because it works even in the presence of activating estrogen receptor mutations, a major mechanism of resistance to aromatase inhibitors. It probably adds to the activity of aromatase inhibitors in treating postmenopausal metastatic disease in tamoxifen-naive populations. I am unaware of any ongoing or planned adjuvant studies with fulvestrant.
The long-term safety of these interventions needs to be defined, especially when treating women with node-negative, hormone receptor–positive, HER2-negative breast cancer, most of whom do not need the therapies beyond a pill a day for 5 or 10 years. At the moment, the costs of CDK4/6 inhibitors and everolimus are orders of magnitude higher than the current cost for tamoxifen, an aromatase inhibitor, or a monthly injection of an LHRH antagonist. Costs may fall because of competition or medical system reforms before we have enough data to routinely recommend any of these medications for the adjuvant treatment of node-negative breast cancer.
What Do We Advise Now?
I favor accepting the major conclusion of TAILORx, and will mention, but not recommend, adjuvant chemotherapy to women with node-negative, hormone receptor–positive, HER2-negative breast cancer with an Oncotype DX recurrence score less than 26.
The evidence that chemotherapy benefits women younger than 50 with recurrence scores of 16 to 25 is shaky because it is based on a subgroup analysis—it should be considered “hypothesis generating.” If one is generating hypotheses, I prefer the 2019 hypothesis that the benefit of chemotherapy in these women is produced by ovarian suppression. I will discuss the benefits, toxicities, and risks of ovarian suppression with premenopausal women aged less than 50 with recurrence scores of 16 to 25, and I will suggest that it is probably less toxic and often more effective than chemotherapy, especially in women under 46, who often retain ovarian function after chemotherapy.
It remains unclear whether the initial hormonal therapy with ovarian function suppression should be tamoxifen followed by an aromatase inhibitor (given for 5 years beginning at menopause) or 5 years of an aromatase inhibitor with later therapy hopefully to be defined by data we do not yet have. In SOFT, the aromatase inhibitor was exemestane. This reduced distant metastases at 8 years by 7% in SOFT and 5% in TEXT among women whose cancers were HER2-negative.9 Many of these women had involved axillary lymph nodes, so the absolute benefits among those with negative nodes (and thus lower risk) will likely be smaller.
The impressive recent rate of progress with hormonal therapy and treatments like CDK4/6 inhibitors and everolimus suggests that we should pair ovarian function suppression with an aromatase inhibitor to “lock in” the initial benefit now—and hope that well-designed clinical trials reported in the interim will tell us what to do next, 5 years later. ■
At Microphone 1 is an occasional column written by Steven E. Vogl, MD, of the Bronx, New York. When he is not in his clinic, Dr. Vogl can generally be found at major oncology meetings and often at the microphone, where he stands ready with critical questions for presenters of new data.
The opinions expressed in this column are those of the author. If you would like to share your opinion on this or another topic, please write to editor@ASCOPost.com.
Acknowledgment: Dr. Vogl thanks Dr. Joseph Sparano, for comments on drafts of this column, and Dr. Robert Gray, the TAILORx statistician, for providing the precise hazard ratios in Tables 1 and 2 as well as 9-year rates of distant metastases for chemoendocrine vs endocrine therapies for women aged < 50 years with recurrence scores of 16 to 25.
DISCLOSURE: Dr. Vogl reported no conflicts of interest.
REFERENCES
1. Sparano JA, et al: Impact of clinical risk category on prognosis and prediction of chemotherapy benefit in early breast cancer by age and the 21-gene recurrence score in TAILORx. 2019 ASCO Annual Meeting. Abstract 503. Presented June 3, 2019.
2. Sparano JA, et al: Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med 380:2395-2405, 2019.
3. Sparano JA, et al: Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 373:2005-2014, 2015.
4. Sparano JA, et al: Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379:111-121, 2018.
5. Cardoso F, et al: 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375:717-729, 2016.
6. Vogl SE: The risks of drug approval based on shaky evidence. The ASCO Post. March 25, 2019.
7. Hudis C, et al: Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: The STEEP system. J Clin Oncol 25:2127-2132, 2007.
8. Vogl SE: TAILORx: How to apply this landmark study. The ASCO Post. August 10, 2018.
9. Vogl SE: Model emphasizes long-term risks of ovarian ablation plus aromatase inhibitor. The ASCO Post. November 10, 2017.
10. Francis PA, et al: Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med 379:122-137, 2018.
11. Vogl SE: 8-year update of SOFT and TEXT trials: Positive but not definitive. The ASCO Post. July 25, 2018.

