
Steven E. Vogl, MD
“At Microphone 1” is an occasional column written by Steven E. Vogl, MD, of Bronx, New York. When he’s not in his clinic, Dr. Vogl can generally be found at major oncology meetings and often at the microphone, where he stands ready with critical questions for presenters of new data. Here Dr. Vogl shares his thoughts on ovarian ablation or suppression in the modern therapy of women with resected breast cancer.
A recently published model of the benefits of ovarian ablation (achieved by laparoscopic bilateral salpingo-oophorectomy) together with an aromatase inhibitor for 5 years, after 5 years of adjuvant tamoxifen, estimates that 12.5% of women will die of complications of early ovarian ablation over 40 years of follow-up.1 This translates to 1,897 additional deaths in an estimated 18,000 hormone receptor–positive women diagnosed up to age 45 in 2016 in the United States, and wipes out a hypothetical survival advantage based on fewer breast cancer and “age-related” deaths. The extra deaths largely occur more than 10 years after ovarian ablation, an effect well described in the Nurses’ Health Study, on which this part of the model is based.2
The model suggests a 10-year mortality of 24.7% with placebo and 22.4% with tamoxifen (both from the ATLAS trial3) and projects a 10-year mortality of 20.5% with ovarian ablation/aromatase inhibitor (based on applying the 4-year hazard rate of 0.82 for extended letrozole after tamoxifen from MA.17 to the ATLAS population).4 After 24.6 years, the tamoxifen and ovarian ablation/aromatase inhibitor survival curves cross, and tamoxifen is the preferred treatment.
The point here is the magnitude of long-term toxicity of ovarian ablation—big enough to wipe out any but very large absolute benefits from ovarian ablation. They have not yet been observed at all.— Steven E. Vogl, MD
Tweet this quote
The model was designed by a group from British Columbia and published in the August 2017 issue of the Journal of the National Comprehensive Cancer Network.1 The relevant paper is as much about cost as it is about benefit, and the model makes many assumptions that extrapolate breast cancer effects far beyond available data. The model prefers laparoscopic bilateral salpingo-oophorectomy to ovarian function suppression (using a gonadotropin-releasing hormone [GnRH] agonist) because the former is less expensive. The point here is the magnitude of long-term toxicity of ovarian ablation—big enough to wipe out any but very large absolute breast cancer benefits from ovarian ablation.
In an earlier paper that modeled 40-year outcomes for the three arms of the SOFT trial, the same group modeled a cohort of the estimated 9,320 women up to 45 years old diagnosed with hormone receptor–positive breast cancer in the United States in 2015 and having prior chemotherapy with preserved ovarian function after the chemotherapy.5 The model predicts 6,690 deaths at 40 years with tamoxifen; 6,075 deaths with ovarian function suppression plus an aromatase inhibitor (OFS/AI); and 5,970 deaths with bilateral salpingo-oophorectomy plus an aromatase inhibitor (BSO/AI) from breast cancer, short-term adverse effects, and “age-associated conditions.” To the latter two predictions, the model adds 577 deaths to the OFS/AI group and 787 deaths to the BSO/AI group from the late effects of ovarian suppression or resection. These extra deaths make tamoxifen the preferred treatment.
Nurses’ Health Study
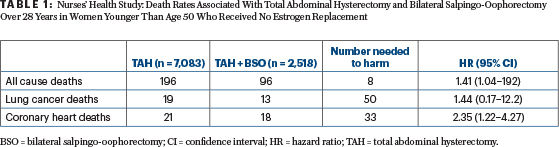
The Nurses’ Health Study is the major, but not the only, basis for attributing excess deaths to premenopausal oophorectomy.2 In 1976, 121,700 female nurses in the United States, aged 30 to 55, were enrolled and followed for 28 years. The relevant paper, among many generated by this huge longitudinal study, sought to ask whether removing the ovaries at the time of hysterectomy for benign disease is good for the patient.
When therapy produces a high rate of lethal toxicity, even distant disease–free survival is not an acceptable surrogate.— Steven E. Vogl, MD
Tweet this quote
Hysterectomy was performed in 30,117 women: alone in 13,203, and with bilateral salpingo-oophorectomy in 16,914. The choice to add oophorectomy was made by patients and physicians, for reasons not known to the study group. The 28-year mortality was 13.3% with hysterectomy and rose to 16.8% when oophorectomy was added—a hazard ratio for death of 1.13 that was statistically significant. As expected, bilateral salpingo-oophorectomy greatly reduced deaths from ovarian cancer, from 44 in the hysterectomy-alone group to 4 when oophorectomy was added.
The relevant subgroup for this analysis consisted of women up to age 50 who never had estrogen therapy. The latter is contraindicated for resected breast cancer for hormone receptor–positive cancers but abrogates the long-term increased mortality from ovarian ablation in the Nurses’ Health Study. Adding bilateral salpingo-oopherectomy in this population increased mortality over 28 years by 41%, with only eight patients subjected to oophorectomy needed to lead to the death of one woman (Table 1).
Interpreting Model Results
The models of Kwon et al1,5 make many assumptions and extrapolations of uncertain validity, and fail to consider the option of continuing tamoxifen until menopause occurs and then giving an aromatase inhibitor for 5 years. There is every reason to assume that the extraordinary activity of an aromatase inhibitor after menopause on tamoxifen seen in the MA.17 trial (a 74% reduction in disease-free survival events4) will apply after 7 or 8 years of tamoxifen, as it did after 5 years.
The quantitative validity of the model results is clearly in doubt. Not in doubt, however, are the long-term risks of oophorectomy and the relatively good prognosis of premenopausal, receptor-positive, resected breast cancer patients in 2017—most of whom will live many years without breast cancer metastases or death.6 We should be concerned about shortening the lives of women cured of their breast cancers.
Chlebowski et al have pointed out that adjuvant ovarian suppression plus an aromatase inhibitor is associated with increased early mortality compared with ovarian suppression plus tamoxifen in each of the three studies looking at this issue (SOFT, TEXT, and ABCSG 12).7 Perhaps the profound estrogen suppression induced by adding an aromatase inhibitor to oophorectomy or ovarian suppression in young women is producing an excess of early deaths from coronary disease, suicide, depression, or some yet undescribed mechanism.
Disease-Free Survival: Poor Surrogate for Overall Survival
The major increase in late deaths among noncancer populations with early bilateral -salpingo-oophorectomy, and indications of increased early deaths among breast cancer patients with ovarian suppression plus aromatase inhibitor therapy, suggests we should insist on favorable overall survival data from randomized clinical trials before advocating an aromatase inhibitor plus either medical ovarian suppression or oophorectomy to our young breast cancer patients.
For breast cancer, disease-free survival is a poor surrogate for overall survival.6 When therapy produces a high rate of lethal toxicity (as may be the case in the first 5 years of adjuvant therapy with an aromatase inhibitor plus ovarian suppression, and as seems to be the case after 25 years for adjuvant aromatase inhibitor therapy plus oophorectomy), even distant disease–free survival is not an acceptable surrogate.
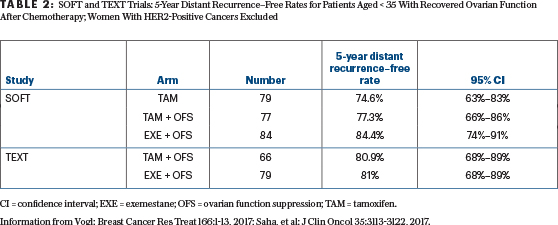
To date, the SOFT and TEXT trials have reported a small improvement in the breast cancer–free interval and disease-free survival, tiny reductions in distant metastases, but no improvement in overall survival from adding ovarian suppression to tamoxifen or from substituting an aromatase inhibitor for tamoxifen with ovarian suppression.8,9 The much-touted benefits of ovarian suppression in the youngest premenopausal “high-risk women” after chemotherapy become very small or vanish when the analysis excludes those with HER2-positive disease and the endpoint is freedom from distant disease (Table 2).9
What to Watch for in San Antonio
Updates of both SOFT and TEXT will be presented in San Antonio in December 2017. It will be important to learn how the patients were treated after the initial 5 years of hormonal therapy, since major benefits can accrue if the right treatments are given in the second 5 years of hormonal therapy.4 They may compensate for an early increase in breast cancer events on tamoxifen without ovarian suppression.
Oncologists should be very interested in whether, with 3 more years of follow-up, there is a continued survival detriment for substituting an aromatase inhibitor for tamoxifen, each with ovarian suppression. If this is the case, adjuvant aromatase inhibitor therapy with ovarian suppression should not be given at all until we get solid data to the contrary.
Careful physicians and worried patients should insist that studies show a significant and sustained improvement in overall survival before advising and accepting adjuvant ovarian suppression, and especially ovarian suppression plus an aromatase inhibitor as part of modern breast cancer adjuvant therapy, even though a recent ASCO guideline recommends it (in high-risk patients).10 ■
Disclaimer: This commentary represents the views of the author and may not necessarily reflect the views of ASCO.
DISCLOSURE: Dr. Vogl reported no conflicts of interest.
REFERENCES
1. Kwon JS, et al: Costs and benefits of extended endocrine strategies for premenopausal breast cancer. J Natl Compr Canc Netw 15:1015-1021, 2017.
2. Parker WH, et al: Long-term mortality associated with oophorectomy compared with ovarian conservation in the Nurses’ Health Study. Obstet Gynecol 121:709-716, 2013.
5. Kwon JS, et al: Long-term consequences of ovarian ablation for premenopausal breast cancer. Breast Cancer Res Treat 157:565-573, 2016.
7. Chlebowski RT, et al: Ovarian suppression in combination endocrine adjuvant therapy in premenopausal women with early breast cancer. Breast Cancer Res Treat 161:185-190, 2017.
8. Francis PA, et al: Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 372:436-446, 2015.
10. Burstein HJ, et al: Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J Clin Oncol 34:1689-1701, 2016.

