In the final per-protocol analysis of the phase III KEYNOTE-811 trial, an overall survival benefit was shown by adding the PD-1 inhibitor pembrolizumab to trastuzumab and chemotherapy in treatment-naive, unresectable, HER2-positive metastatic gastric or gastroesophageal junction adenocarcinoma.1

Sara Lonardi, MD
“KEYNOTE-811 has met prespecified criteria for significance at all endpoints evaluated in the whole population, for objective response rate, progression-free survival, and overall survival,” said Sara Lonardi, MD, of the Veneto Institute of Oncology in Padua, Italy.
In the study of 698 patients, at a median follow-up of 50.2 months (1 year past the previous analysis), pembrolizumab added to trastuzumab and chemotherapy in the first-line setting led to a median overall survival of 20.0 months vs 16.8 months for placebo plus trastuzumab and chemotherapy (hazard ratio [HR] = 0.80; P = .0040). The 36-month overall survival rate was 28% with pembrolizumab and 23% with placebo.
“The curves separate early and stay parallel for all of the time, with an absolute gain at the prespecified points of 6% at 12 months and 5% at 24 and 36 months,” Dr. Lonardi said. Patients with PD-L1 expression (combined positive score [CPS] ≥ 1) had similar benefit as the overall population (HR = 0.79; 95% confidence interval [CI] = 0.66–0.95)—whereas the small group of patients with PD-L1 CPS < 1 did not benefit from the addition of immunotherapy.
The findings of KEYNOTE-811 further establish pembrolizumab plus chemotherapy as the first-line standard of care in PD-L1–positive advanced HER2-positive gastric or gastroesophageal junction adenocarcinoma, Dr. Lonardi said.
About KEYNOTE-811
The study randomly assigned 698 patients with advanced gastric or gastroesophageal junction adenocarcinoma to receive pembrolizumab (n = 350) or placebo (n = 348), plus trastuzumab and chemotherapy, which was capecitabine and oxaliplatin (CAPOX) in 85% and fluorouracil plus cisplatin in the others. In both arms, 85% of patients had PD-L1 CPS ≥ 1.
Progression-free survival at the final analysis mirrored the data reported at the interim analysis,2 according to Dr. Lonardi, noting those findings led to the pembrolizumab plus chemotherapy regimen to be considered as the first-line standard of care. In the current analysis, median progression-free survival was 10.0 months with pembrolizumab compared with 8.1 months with trastuzumab and chemotherapy alone (HR = 0.73; 95% CI = 0.61–0.87), with 18% and 11% of patients, respectively, free of disease progression at 36 months. In the second interim analysis, progression-free survival was 10.0 vs 8.1 months, respectively (HR = 0.72; P = .0002).
Overall response rates were 72.6% with pembrolizumab and 60.1% without it, which Dr. Lonardi called a “nice gain” of 12.6% with the PD-1 inhibitor and prolongation of duration of response, which was 11.3 months vs 9.5 months, respectively. At 36 months, responses were continuing for 24% of patients in the pembrolizumab arm and 15% in the placebo group.
Most key subgroups derived a similar -benefit from pembrolizumab, although there were some “qualitative differences,” Dr. Lonardi noted. Asian patients—34% of the population—had no significant benefit (HR = 1.05; 95% CI = 0.77–1.43) than the non-Asian population (HR = 0.72; 95% CI = 0.59–0.87), but the most important difference was observed by PD-L1 CPS expression level. Patients with a PD-L1 CPS ≥ 1 experienced a 21% reduction in the risk of death and a 28% reduction in disease progression with pembrolizumab, whereas the CPS < 1 subset (15% of the population) derived no benefit (Table 1). Median overall survival gain for patients with a CPS ≥ 1 receiving pembrolizumab was higher than for the overall population.
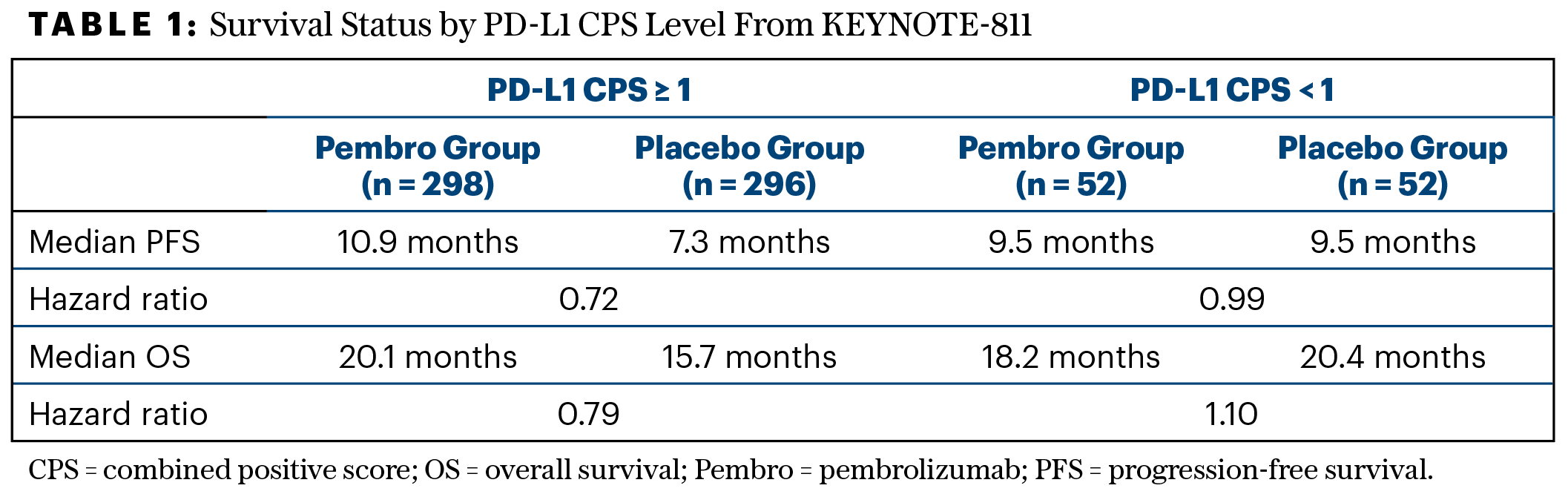
“The data for CPS ≥ 1 are solid, confirming the gain in progression-free and overall survival, although that for CPS < 1 is obviously affected by the small sample size,” she said.
The adverse event profile remained unchanged at the final analysis. Serious adverse events were noted for 26% of the pembrolizumab arm compared with 23% of the control group. The drug (or placebo) was discontinued by 37% and 34%, respectively. The most common treatment-related toxicities were diarrhea, nausea, and anemia, which were primarily the result of the chemotherapy component. No new immune-mediated concerns were reported.
EXPERT POINT OF VIEW

Filippo Pietrantonio, MD
The invited discussant of -KEYNOTE-811 was Filippo Pietrantonio, MD, of the Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy, who noted the previous positive findings that led to regulatory approval of this regimen. “In the final analysis, the co-primary endpoint of overall survival was finally met…. This is the first time that formal testing has been performed on overall survival, and the fact that patients gained around 3 months is clinically meaningful,” he declared. “Despite the discontinuation of pembrolizumab after 2 years, it provided an absolute gain in long-term survival of at least 5%, and this is on top of the benefit of anti-HER2 therapy.”
Dr. Pietrantonio continued: “The overall survival benefit—4.4 months gained—was even greater in the large subgroup with a PD-L1 combined positive score [CPS] ≥ 1, and this fits with current approved indications. In HER2-positive disease, for patients with a CPS ≥ 1, the addition of pembrolizumab to trastuzumab and chemotherapy is strongly recommended, whereas in patients with a CPS < 1, trastuzumab and chemotherapy remains the standard of care.”
Further elaborating on the importance of PD-L1 status, Dr. Pietrantonio indicated that KEYNOTE-859, which evaluated pembrolizumab in HER2-negative gastric cancer, examined the benefit according to CPS levels of ≥ 1, 1 to 9, and ≥ 10 and found the most benefit in the ≥ 10 subset.3 “This highlights that CPS ≥ 1 is a highly heterogeneous subgroup, likely regardless of HER2 status,” he commented.
As the treatment landscape is rapidly evolving for HER2-positive gastric cancer—with many novel combinations now in clinical trials—the standard-of-care comparator arm should now be pembrolizumab, trastuzumab, and chemotherapy, Dr. Pietrantonio maintained. “As a next step, it will be important to test the KEYNOTE-811 combination in earlier-stage gastric or gastroesophageal junction adenocarcinoma to help move on from chemotherapy as the current standard of care.”
DISCLOSURE: Dr. Lonardi reported personal honoraria as an invited speaker from Amgen, AstraZeneca, Bristol Myers Squibb, Incyte, GSK, Lilly, Merck Serono, MSD, Pierre-Fabre, Roche, and Servier; participation in an advisory board for Amgen, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Daiichi Sankyo, GSK, Incyte, Lilly, Merck Serono, MSD, Servier, Takeda, Rottapharm, and BeiGene. Dr. Pietrantonio reported financial relationships with BMS, MSD, Amgen, Merck Serono, Pierre-Fabre, Servier, Bayer, Takeda, Astellas, Johnson & Johnson, Rottapharm Biotech, Ipsen, AstraZeneca, GSK, Daiichi Sankyo, Seagen/Pfizer, BeiGene, and Jazz Pharmaceuticals.
REFERENCES
1. Janjigian YY, Kawazoe A, Bai Y, et al: Final overall survival for the phase III, KEYNOTE-811 study of pembrolizumab plus trastuzumab and chemotherapy for HER2+ advanced, unresectable or metastatic G/GEJ adenocarcinoma. ESMO Congress 2024. Abstract 1400O. Presented September 14, 2024.
2. Janjigian YY, Kawazoe A, Bai Y, et al: Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: Interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet 402:2197-2208, 2023.
3. Rha SY, Oh DY, Yañez P, et al: Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 24:1181-1195, 2023.

