The benefit of anti–PD-1 antibodies in the adjuvant treatment of patients with stage III or stage IV melanoma continues to be observed at around 4 years for both pembrolizumab and nivolumab, according to updates of pivotal trials presented at the European Society for Medical Oncology (ESMO) Virtual Congress 2020.

Rodabe N. Amaria, MD
“It’s safe to say the scales have been shifted in the adjuvant treatment of advanced melanoma,” Rodabe N. Amaria, MD, of The University of Texas MD Anderson Cancer Center, Houston, said as the invited discussant of KEYNOTE-054 and CheckMate 238. “Now we have regimens that are really prioritized for improving patient outcomes with significant minimization of toxicity.”
Pembrolizumab conveyed a 40% reduction in the risk of distant metastases vs placebo in patients with stage III melanoma, whereas nivolumab reduced the risk of recurrence by 29% vs the active comparator, ipilimumab, in stage III or IV disease, investigators reported.
KEYNOTE-054: Study Details
During ESMO 2020, Alexander Eggermont, MD, Chief Scientific Officer at the Princess Maxima Center in Utrecht, Netherlands, presented the final distant

Alexander Eggermont, MD
metastasis–free survival analysis (a secondary endpoint) for KEYNOTE-054.1 Data for recurrence-free survival, the primary endpoint, were presented previously at the ASCO20 Virtual Scientific Program.2
In the phase III KEYNOTE-054 trial, 1,019 adults with resected stage IIIA, IIIB, or IIIC melanoma were randomly assigned to pembrolizumab (200 mg every 3 weeks for 18 doses) or placebo until disease recurrence or unacceptable toxicity. After 1 year of treatment, patients who experienced disease progression on placebo could receive pembrolizumab in the metastatic setting; pembrolizumab-treated patients with recurrence more than 6 months after adjuvant therapy could also receive more pembrolizumab. This design is intended to determine whether pembrolizumab is as effective if given after recurrence.
KEYNOTE-054: Improvements in Key Secondary Endpoint
As reported by Dr. Eggermont, the 3.5-year distant metastasis–free survival rates were 65.3% with adjuvant pembrolizumab and 49.4% with placebo (hazard ratio [HR] = 0.60; P < .0001). By type of first recurrence, distant metastasis was seen in 25.0% of the pembrolizumab arm and 39.5% of the placebo arm (HR = 0.58; P < .001); locoregional recurrences were observed in 14.0% and 18.9%, respectively (HR = 0.73; P = .050).
A sustained recurrence-free survival benefit was seen across all stages at 3.5 years, with recurrence-free survival rates of 59.8% with pembrolizumab vs 41.4% with placebo (HR = 0.59; P < .001). The benefit in distant metastasis–free and recurrence-free survival was consistent across all stages and across key subgroups, including disease stage, BRAF mutation status, and PD-L1 expression (Table 1).
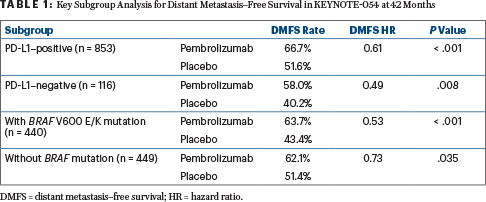
“These new and updated data, including first-time results for distant metastasis–free survival, are significant, showing that adjuvant pembrolizumab not only delayed recurrence, but also delayed distant metastasis, further reinforcing the benefits of pembrolizumab for patients with stage III melanoma,” Dr. Eggermont said.
Overall survival results are not mature; events are occurring slowly because of the crossover design and the use of additional effective lines of treatment. “We do not even expect to be able to report on overall survival within 4 years from now,” he noted.
CheckMate 238: Study Details
CheckMate 238 enrolled 906 patients who had undergone complete resection of stage IIIB/C or IV melanoma.3 Patients were randomly assigned to receive nivolumab at 3 mg/kg every 2 weeks or ipilimumab at 10 mg/kg every 3 weeks; they were given four doses, then dosed every 12 weeks (from week 24) for up to 1 year. The primary endpoint was recurrence-free survival. Distant metastasis–free survival in patients with stage III disease was an exploratory endpoint.
“Nivolumab continues to be an effective adjuvant treatment for patients with resected high-risk melanoma at 4 years, with sustained recurrence-free and distant

Jeffrey Weber, MD, PhD
metastasis–free survival benefit vs the active comparator ipilimumab,” said Jeffrey Weber, MD, PhD, Deputy Director and Co-Director of the Melanoma Program at the Laura and Isaac Perlmutter Cancer Center at New York University–Langone Health, New York.
CheckMate 238: Key Outcomes
After 48 months of follow-up, updated median recurrence-free survival was 52.4 months with nivolumab and 24.1 months with ipilimumab (HR = 0.71; P = .0003). The 4-year recurrence-free survival rates were 52% and 41%, respectively, and all prespecified subgroups benefited more from nivolumab (Table 2). In most comparisons, more than an 8% absolute difference in recurrence rates was observed, Dr. Weber reported. “The superiority of nivolumab was maintained,” he added.
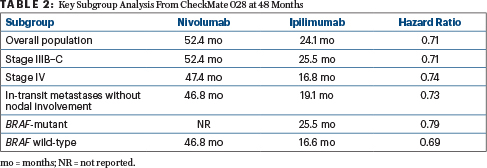
As for distant metastasis–free survival in patients with stage IIIB–C disease, at 48 months the median had not been reached with nivolumab and was 52.9 months with ipilimumab (HR = 0.79; P = .045). In the first report of overall survival for an adjuvant anti–PD-1 regimen, median overall survival was not reached in either arm. In addition, according to Dr. Weber, events (n = 211) were lower than anticipated (n = 302).
KEY POINTS
- After 42 months of follow-up, adjuvant pembrolizumab conveyed a 40% reduction in the risk of distant metastasis in the phase III KEYNOTE-054 trial of patients with resected stage III melanoma.
- After 48 months of follow-up, adjuvant nivolumab conveyed a 29% reduction in the risk of recurrence in patients with stage III or IV melanoma in the phase III CheckMate 238 trial.
- Virtually all subsets of patients benefited from adjuvant immunotherapy.
At 4 years, 78% of patients on the nivolumab arm and 77% on the ipilimumab arm were still alive. The similar outcome is presumably a result of more post-relapse immunotherapy received in the ipilimumab arm (57% vs 49%), he said.
In both KEYNOTE-054 and CheckMate 238, late-emergent treatment-related adverse events were consistent with the established safety profiles of the drugs.
DISCLOSURE: KEYNOTE-054 was funded by Merck & Co. CheckMate 238 was funded by Bristol Myers Squibb and Ono Pharmaceutical. Dr. Amaria has received institutional research funding from Array BioPharma, Bristol Myers Squibb, Genentech, Iovance Biotherapeutics, Merck, and Novartis. Dr. Eggermont holds stock or other ownership interests in RiverD, Skyline Diagnostics, and Theranovir; has received honoraria from BioCad, BioInvent, CatalYm, Ellipses Pharma, GlaxoSmithKline, IO Biotech, ISA Pharmaceuticals, MSD, Nektar, Novartis, Pfizer, Sellas Life Sciences, and Skyline Diagnostics; has served as a consultant or advisor to BioInvent, CatalYm, Ellipses Pharma, GlaxoSmithKline, IO Biotech, ISA Pharmaceuticals, MSD, Nektar, Novartis, Pfizer, Sellas Life Sciences, and Skyline Diagnostics; has participated in a speakers bureau for BioCad and MSD; has provided expert testimony on behalf of Novartis; and has been reimbursed for travel, accommodations, or other expenses by Bristol Myers Squibb. Dr. Weber holds stock or other ownership interests in Biond, CytomX Therapeutics, Immunimax, and Protean Biodiagnostics; has received honoraria from Amgen, AstraZeneca, Bristol Myers Squibb, Celldex, CytomX Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Jounce Therapeutics, Kirin Pharmaceuticals, Merck, Moderna Therapeutics, Novartis, Regeneron, Roche, Sellas Life Sciences, Takeda, and WindMIL; has served as a consultant or advisor to Amgen, AstraZeneca, Bristol Myers Squibb, Celldex, CytomX Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Jounce Therapeutics, Kirin Pharmaceuticals, Merck, Moderna Therapeutics, Novartis, Protean Biodiagnostics, Roche, Sellas Life Sciences, and WindMIL; has received institutional research funding from Astellas Pharma, Bristol Myers Squibb, Genentech, GlaxoSmithKline, Incyte, Merck, NextCure, Novartis, and Roche; holds intellectual property for “41BB induced TIL,” an “ipilimumab biomarker,” and a “PD-1 antibody biomarker”; and has been reimbursed for travel, accommodations, or other expenses by Amgen, AstraZeneca, Bristol Myers Squibb, Celldex, Genentech, GlaxoSmithKline, Merck, Novartis, and Roche.
REFERENCES
1. Eggermont AM, Blank CU, Mandalà M, et al: Pembrolizumab vs placebo after complete resection of high-risk stage III melanoma: Final results regarding distant metastasis-free survival from the EORTC 1325-MG/KEYNOTE-054 double-blinded phase III trial. 2020 ESMO Virtual Congress. Abstract LBA46. Presented September 19, 2020.
2. Eggermont AM, Blank CU, Mandalà M, et al: Pembrolizumab vs placebo after complete resection of high-risk stage III melanoma: New recurrence-free survival results from the EORTC 1325-MG/Keynote 054 double-blinded phase III trial at three-year median follow-up. 2020 ASCO Virtual Scientific Program. Abstract 10000.
3. Weber J, Del Vecchio M, Mandalàa M, et al: Adjuvant nivolumab vs ipilimumab in resected stage III/IV melanoma: 4-year recurrence-free and overall survival results from CheckMate 238. 2020 ESMO Virtual Congress. Abstract 1076O. Presented September 19, 2020.

