
Nizar M. Tannir, MD, FACP

Andrew W. Hahn, MD

Pavlos Msaouel, MD, PhD
At the 2021 Genitourinary Cancers Symposium, Motzer et al presented the clinical results of the CLEAR trial, adding a novel regimen, lenvatinib plus pembrolizu-mab, to the growing armamentarium of first-line treatments for patients with metastatic clear cell renal cell carcinoma (RCC). The findings have been reported by the authors in The New England Journal of Medicine1 and are reviewed in this issue of The ASCO Post.
CLEAR was a phase III clinical trial that randomly assigned 1,069 patients with treatment-naive metastatic RCC to lenvatinib at 20 mg daily plus pembrolizumab at 200 mg every 3 weeks, lenvatinib at 18 mg daily plus everolimus 5 mg daily, or sunitinib 50 mg daily (4 weeks on, 2 weeks off) in a 1:1:1 ratio.1 CLEAR met its primary endpoint, with lenvatinib plus pembrolizumab significantly improving progression-free survival compared to sunitinib (hazard ratio [HR] = 0.39, 95% confidence interval [CI] = 0.32–0.49; median = 23.9 vs 9.2 months). Lenvatinib plus pembrolizumab also improved the objective response rate compared to sunitinib (71.0% vs 36.1%) with an impressive complete response rate of 16.1%. Overall survival was also significantly longer with lenvatinib plus pembrolizumab than sunitinib (HR = 0.66, 95% CI = 0.49–0.88). In the third arm, lenvatinib plus everolimus significantly improved progression-free survival compared to sunitinib (median = 14.7 vs 9.2 months, HR = 0.65, 95% CI = 0.53–0.83), but overall survival benefit was inconclusive (HR = 1.15, 95% CI = 0.88–1.50).
The Kaplan-Meier curve for overall survival generated discussion because the survival curves for lenvatinib plus pembrolizumab and sunitinib crossed at approximately 34 months. However, these -Kaplan-Meier curves can be misleading for at least two reasons. First, at the 30-month time point onwards, more than 80% of patients from each treatment group have either died or are censored, resulting in substantial imprecision of the Kaplan-Meier estimates—which is the reason it has been proposed to refrain from showing Kaplan-Meier curves when only around 10% to 20% of patients remain in follow-up.2 Second, the presented Kaplan-Meier curves are marginal estimates that are less precise and less likely to satisfy the proportional hazards assumption than the stratified log rank and Cox regression models used to generate all the major statistical estimates of the CLEAR trial.3
Tolerability of Starting Dose in Question
The regimen of lenvatinib at 18 mg daily plus everolimus at 5 mg daily was approved in 2016 as second-line therapy for patients who experienced progressive disease after one prior antiangiogenic therapy.4,5 When using this regimen in the second-line and beyond setting, dose reductions of lenvatinib are common, so the 20-mg daily dose of lenvatinib raises questions about the tolerability of this starting dose. In CLEAR, 68.8% of patients receiving lenvatinib plus pembrolizumab required dose reductions due to treatment-related adverse events compared to 50.3% with sunitinib. For the patients receiving lenvatinib plus pembrolizumab, 25.6% discontinued lenvatinib due to treatment-related adverse events, 28.7% discontinued pembrolizumab, and 13.4% discontinued both lenvatinib and pembrolizumab. Although lenvatinib plus pembrolizumab is a highly active regimen, lenvatinib starting at 20 mg daily appears to be difficult to tolerate for many patients, and we do not know how starting lenvatinib at a reduced dose would impact the efficacy of lenvatinib plus pembrolizumab. In a phase II trial of 343 patients who had progressive disease on prior antiangiogenic therapy, the lenvatinib starting dose of 14 mg daily combined with everolimus at 5 mg daily yielded a numerically lower overall response rate and a shorter progression-free survival than the lenvatinib starting dose of 18 mg daily in combination with everolimus at 5 mg daily.6
Cross-Trial Comparisons
At this time, the U.S. Food and Drug Administration has not approved lenvatinib plus pembrolizumab. We anticipate that it will be approved in the near future and, when approved, it will become the fifth immuno-oncology combination for the first-line treatment of metastatic RCC, in addition to nivolumab plus ipilimumab, pembrolizumab plus axitinib, avelumab plus axitinib, and nivolumab plus cabozantinib (Table 1).1,7-12
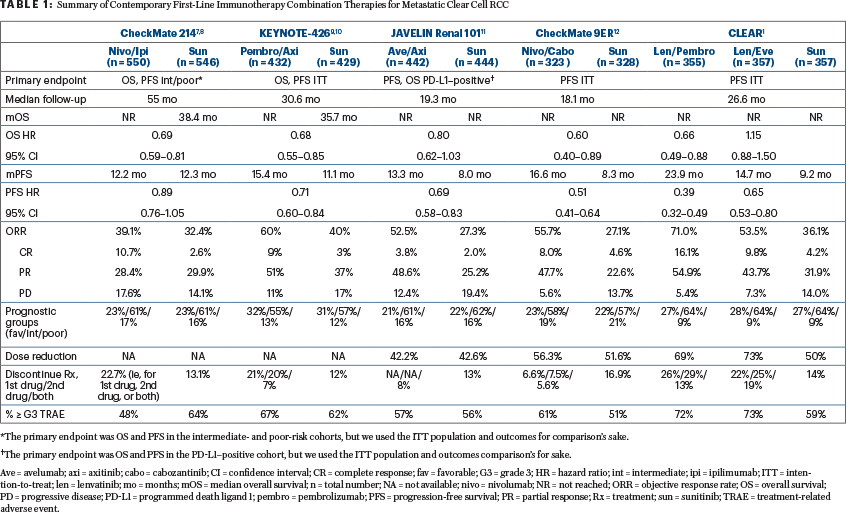
It is often, appropriately, noted that cross-trial comparisons have multiple serious caveats. However, clinicians are instinctively inclined to compare trials, in large part because such comparisons are contingent on assumptions that are not fundamentally different from the assumptions used in daily practice to generalize or transport inferences from a randomized controlled trial to a specific patient seen in clinic, who will often have little in common with the trial population.13,14
What is helpful to oncologists who treat patients with RCC is that all the first-line immuno-oncology combination trials to date have used the same comparator (sunitinib) and treated broadly similar populations (Table 1). When comparing treatment options for a specific patient in clinic, we should always be mindful that we are making decisions under uncertainty while relying on imperfect data and assumptions—ie, the natural habitat for any practicing clinician. Our decisions should ideally be informed by the totality of the available evidence, capitalizing on the natural synergy between randomized and real-world observational data.15
Beware, however, of the most misleading graph in contemporary oncology: forest plots. These plots were originally developed for meta-analysis of randomized controlled trials16 but have more recently become a source of much confusion when used for subgroup analyses in single randomized controlled trials. Case in point: all the forest plots presented from the pivotal immunotherapy combination trials listed in Table 1 tell us only that there is a remarkable constancy of the hazard ratios across patient subgroups. This should not be very surprising since this constancy is a fundamental assumption of many applied statistical models. Instead of looking for subgroup-specific differences in the hazard and odds ratio scales represented by forest plots, patient-specific inferences can be much more informed by considering outcome heterogeneity using prognostic information derived from both randomized clinical trial and observational data.17,18
Choosing a First-Line Regimen: Choices Abound
The selection of first-line treatment for metastatic RCC should be guided by individual patient characteristics combined with a vision for treatment sequencing to optimize survival. An essential question to consider when selecting a first-line regimen is whether the patient would be a good candidate for the CTLA-4 inhibitor ipilimumab. The OMNIVORE, TITAN-RCC, and HCRN GU16-260 studies suggested that ipilimumab has limited activity after disease progression on immunotherapy-based regimens, so if ipilimumab is a desired component of the treatment sequence, it should preferably be administered as first-line in combination with nivolumab.19-21
In regard to sequencing subsequent lines of therapy, cabozantinib has activity as a single agent after disease progression on nivolumab plus ipilimumab or pembrolizumab plus axitinib.22-24 The activity of cabozantinib after progression on lenvatinib plus pembrolizumab is unknown, and the overlapping spectrum of tyrosine kinases targeted by cabozantinib and lenvatinib raises concern for cross-resistance. Retrospective data suggest, however, that lenvatinib plus everolimus has activity in patients after progressive disease on immunotherapy-based regimens and cabozantinib.25 Thus, for clinicians who are comfortable managing immune-mediated adverse events, first-line nivolumab plus ipilimumab might be preferred in patients with International Metastatic RCC Database Consortium intermediate- or poor-risk disease who do not have an urgent need for reduction in tumor volume. We believe ipilimumab is a key component of immunotherapy-based therapy, responsible for the durability of major responses and contributing to the potential for a meaningful treatment-free survival.26 First-line nivolumab plus ipilimumab is also currently our preferred treatment for patients with advanced clear cell RCC and sarcomatoid dedifferentiation based on the encouragingly high complete response rate (18.9%) and favorable long-term survival outcomes.27
First-line immunotherapy/tyrosine kinase inhibitor regimens can be selected for patients who have an urgent need for reduction in tumor volume or have highly symptomatic disease, especially if they may not get a chance to receive a second-line therapy or have preexisting autoimmune disease precluding anti–CTLA-4 therapy. In these settings, lenvatinib plus pembrolizumab should be considered for patients with a strong desire for a complete response, irrespective of toxicity, or who need an urgent response yet can still tolerate lenvatinib at 20 mg daily. In contrast, pembrolizumab plus axitinib or nivolumab plus cabozantinib are suited for clinicians seeking a well-tolerated immunotherapy/tyrosine kinase inhibitor regimen, with nivolumab plus cabozantinib having demonstrated notable health-related quality of life benefits over sunitinib.12
The inconclusive overall survival results of avelumab plus axitinib have limited its use in metastatic RCC, compared with the other immunotherapy combinations.11 Patients with favorable-risk metastatic RCC have many options to consider including active surveillance, surgical resection, or stereotactic body radiation for oligometastatic disease, single-agent VEGFR tyrosine kinase inhibitor, or immunotherapy combinations.28,29 For those patients who cannot receive any immunotherapy, lenvatinib plus everolimus may become a reasonable new first-line option, if approved, and it is a particularly attractive therapy for patients with a history of solid organ transplantation due to the immunomodulatory effects of mTOR inhibition.
‘Powerful’ New Combination for Metastatic RCC
In sum, lenvatinib plus pembrolizumab adds a highly active immunotherapy-based regimen to the first-line treatment of metastatic RCC. Clinicians must consider whether the excellent efficacy of lenvatinib plus pembrolizumab justifies the toxicity of lenvatinib at 20 mg daily and the questions surrounding treatment sequencing. For these first-line immunotherapy combination doublets, long-term follow-up is necessary to identify the fraction of patients who will achieve durable responses to treatment. With the advent of the powerful lenvatinib plus pembrolizumab combination, we may be approaching the efficacy ceiling for doublet combinations in unselected patients with metastatic RCC. Future efforts in this space should explore the value of triplet combinations and biologically driven patient selection.30-33
Drs. Hahn, Msaouel, and Tannir work in the Department of Genitourinary Medical Oncology, Division of Cancer Medicine at The University of Texas MD Anderson Cancer Center, Houston.
DISCLOSURE: Dr. Hahn reported no conflicts of interest. Dr. Msaouel has received honoraria from Bristol Myers Squibb/Medarex, Exelixis, Mirati Therapeutics, and Pfizer; and has received institutional research funding from Bristol Myers Squibb, Gateway for Cancer Research, Mirati Therapeutics, and Takeda. Dr. Tannir has received honoraria from Bristol Myers Squibb, Calithera Biosciences, Eisai Medical Research, Eli Lilly, Exelixis, Ipsen, Merck Sharp & Dohme, Nektar, Neoleukin Therapeutics, Novartis, Oncorena, Ono Pharmaceutical, Pfizer, and Surface Oncology; has served in a consulting or advisory role for Bristol Myers Squibb, Calithera Biosciences, Eisai Medical Research, Eli Lilly, Exelixis, Ipsen, Merck Sharp & Dohme, Nektar, Neoleukin Therapeutics, Novartis, Oncorena, Ono Pharmaceutical, Pfizer, and Surface Oncology; has received research funding from Arrowhead Pharmaceuticals, Bristol Myers Squibb, Calithera Bioscience, Eisai, Epizyme, Exelixis, Lilly, Mirati Therapeutics, Nektar Therapeutics, Pfizer, and Takeda; and has been reimbursed for travel, accommodations, or other expenses by Bristol Myers Squibb, Calithera Biosciences, Eisai Medical Research, Ipsen, Lilly Oncology, Nektar, Pfizer, and Surface Oncology.
REFERENCES
2. Pocock SJ, Clayton TC, Altman DG: Survival plots of time-to-event outcomes in clinical trials: Good practice and pitfalls. Lancet 359:1686-1689, 2002.
3. Cox DR: Regression models and life-tables. J R Stat Soc Series B Stat Methodol 34:187-220, 1972.
4. Motzer RJ, Hutson TE, Glen H, et al: Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol 16:1473-1482, 2015.
5. Motzer RJ, Hutson TE, Ren M, et al: Independent assessment of lenvatinib plus everolimus in patients with metastatic renal cell carcinoma. Lancet Oncol 17:e4-e5, 2016.
6. Pal SK, Puente J, Heng DY, et al: Phase 2 trial of lenvatinib at 2 starting doses plus everolimus in renal cell carcinoma. International Kidney Cancer Symposium 2020. Poster 33. Presented November 6-7, 2020.
7. Motzer RJ, Tannir NM, McDermott DF, et al: Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277-1290, 2018.
8. Albiges L, Tannir NM, Burotto M, et al: Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 5:e001079, 2020.
9. Rini BI, Plimack ER, Stus V, et al: Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1116-1127, 2019.
10. Powles T, Plimack ER, Soulières D, et al: Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol 21:1563-1573, 2020.
11. Choueiri TK, Motzer RJ, Rini BI, et al: Updated efficacy results from the JAVELIN Renal 101 trial: First-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol 31:1030-1039, 2020.
12. Choueiri TK, Powles T, Burotto M, et al: Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 384:829-841, 2021.
13. Dahabreh IJ, Hernán MA: Extending inferences from a randomized trial to a target population. Eur J Epidemiol 34:719-722, 2019.
14. Elwood JM: Commentary: On representativeness. Int J Epidemiol 42:1014-1015, 2013.
15. Lyman GH, Kuderer NM: Randomized controlled trials versus real-world data in the COVID-19 era: A false narrative. Cancer Invest 38:537-542, 2020.
16. Chia PL, Gedye C, Boutros PC, et al: Current and evolving methods to visualize biological data in cancer research. J Natl Cancer Inst 108:djw031, 2016.
17. Kent DM, Steyerberg E, van Klaveren D: Personalized evidence based medicine: Predictive approaches to heterogeneous treatment effects. BMJ 363:k4245, 2018.
18. Kent DM, Paulus JK, van Klaveren D, et al: The predictive approaches to treatment effect heterogeneity (PATH) statement. Ann Intern Med 172:35-45, 2020.
19. McKay RR, McGregor BA, Xie W, et al: Optimized management of nivolumab and ipilimumab in advanced renal cell carcinoma: A response-based phase II study (OMNIVORE). J Clin Oncol 38:4240-4248, 2020.
20. Grimm MO, Schmidinger M, Duran Martinez I, et al: Tailored immunotherapy approach with nivolumab in advanced renal cell carcinoma (TITAN-RCC). Ann Oncol 30(suppl 5):V892, 2019.
21. Atkins MB, Jegede O, Haas NB, et al: Phase II study of nivolumab and salvage nivolumab + ipilimumab in treatment-naive patients (pts) with advanced renal cell carcinoma (RCC) (HCRN GU16-260). J Clin Oncol 38(suppl):5006, 2020.
22. Shah AY, Kotecha RR, Lemke EA, et al: Outcomes of patients with metastatic clear-cell renal cell carcinoma treated with second-line VEGFR-TKI after first-line immune checkpoint inhibitors. Eur J Cancer 114:67-75, 2019.
23. Ged Y, Gupta R, Duzgol C, et al: Systemic therapy for advanced clear cell renal cell carcinoma after discontinuation of immune-oncology and VEGF targeted therapy combinations. BMC Urol 20:84, 2020.
24. McGregor BA, Lalani AA, Xie W, et al: Activity of cabozantinib after immune checkpoint blockade in metastatic clear-cell renal cell carcinoma. Eur J Cancer 135:203-210, 2020.
25. Wiele A, Ross J, Chahoud J, et al: Lenvatinib alone or in combination with everolimus in heavily pretreated patients with metastatic renal cell carcinoma immune checkpoint inhibitors. Ann Oncol 30(suppl 5):V390, 2019.
26. Regan M, Jegede O, Mantia C, et al: Treatment-free survival, with and without toxicity, after immuno-oncology vs targeted therapy for advanced renal cell carcinoma (aRCC): 42-month results of CheckMate 214. Ann Oncol 31 (suppl 4):S561, 2020.
27. Tannir NM, Signoretti S, Choueiri TK, et al: Efficacy and safety of nivolumab plus ipilimumab versus sunitinib in first-line treatment of patients with advanced sarcomatoid renal cell carcinoma. Clin Cancer Res 27:78-86, 2021.
28. Rini BI, Dorff TB, Elson P, et al: Active surveillance in metastatic renal-cell carcinoma: A prospective, phase 2 trial. Lancet Oncol 17:1317-1324, 2016.
29. Hannan R, Christensen M, Robles L, et al: Phase II trial of stereotactic ablative radiation (SAbR) for oligometastatic kidney cancer. J Clin Oncol 39(suppl):311, 2021.
30. Choueiri TK, Albiges L, Powles T, et al: A phase III study (COSMIC-313) of cabozantinib (C) in combination with nivolumab (N) and ipilimumab (I) in patients (pts) with previously untreated advanced renal cell carcinoma (aRCC) of intermediate or poor risk. J Clin Oncol 38(suppl):TPS767, 2020.
31. Msaouel P, Gao J, Yuan Y, et al: Phase I/IB trial of sitravatinib (Sitra) + nivolumab (Nivo) + ipilimumab (Ipi) in patients (pts) with advanced clear cell renal cell carcinoma (accRCC) or other solid malignancies. J Clin Oncol 39(suppl):TPS365, 2021.
32. Motzer RJ, Robbins PB, Powles T, et al: Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: Biomarker analysis of the phase 3 JAVELIN Renal 101 trial. Nat Med 26:1733-1741, 2020.
33. McDermott DF, Huseni MA, Atkins MB, et al: Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 24:749-757, 2018.


