In the phase III DETERMINATION trial, progression-free survival was significantly improved with triplet induction therapy and early transplantation in newly diagnosed patients with multiple myeloma, but overall survival at 5 years was similar to the nontransplant approach.1 The findings were presented at the Plenary Session of the 2022 ASCO Annual Meeting by Paul G. Richardson, MD, Clinical Program Leader and Director of Clinical Research at the Jerome Lipper Multiple Myeloma Center at Dana-Farber Cancer Institute and the RJ Corman Professor of Medicine at Harvard Medical School, Boston.1 They were simultaneously published in The New England Journal of Medicine.2

Paul G. Richardson, MD
“The addition of transplant to novel agent triplet induction therapy using lenalidomide, bortezomib, and dexamethasone [RVd] followed by lenalidomide maintenance until disease progression resulted in a highly significant increase in progression-free survival, with an improvement in the median of over 21 months. Remarkably, despite this advantage, overall survival with follow-up that is now approaching a median of 7 years proved similar between the two arms,” Dr. Richardson reported.
“Our results provide support for personalized treatment approaches,” Dr. Richardson said. “Importantly, we also saw more toxicity and significant effects on quality of life with early transplant. Overall, these results suggest you may be able to selectively delay transplant to use it in a more tailored fashion, recognizing there is an impressive progression free survival benefit, but to date there appears to be equal outcome in overall survival.” He continued, “patients therefore have the option of keeping early transplant in reserve, particularly if they have a high-quality response to induction therapy and especially in the setting of MRD [measurable residual disease] negativity.”
Primary Endpoint Met
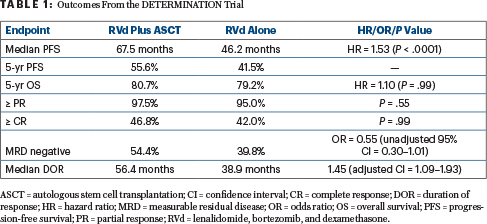
After a median follow-up of 76.0 months, median progression-free survival was 67.5 months with RVd and autologous stem cell transplantation (ASCT), vs 46.2 months with RVd alone (hazard ratio [HR] = 1.53; P < .0001). At 5 years, overall survival was 80.7% vs 79.2%, respectively (HR = 1.10; P = .99). See Table 1 for these and other outcomes from the DETERMINATION trial.
Although response rates and quality of responses were similar between the arms, “interestingly and encouragingly,” he added, MRD negativity was improved with transplantation. The advantages of RVd plus ASCT, however, came at the cost of more hematologic toxicity and nonhematologic side effects, as well as certain secondary cancers.
The lack of an overall survival benefit is probably associated with the many highly effective options now available after first-line therapy, Dr. Richardson suggested. After RVd alone, 28% of participants had ASCT as salvage therapy; other treatments included next-generation immunomodulatory drugs, proteasome inhibitors, and monoclonal antibodies.
By cytogenetic risk, median progression-free survival was 82.3 months with transplant vs 53.2 months without, respectively, for the standard-risk group. Importantly, for patients with high-risk cytogenetics, it was 55.5 vs 17.1 months, respectively. Patients with t(4;14) mutations derived more progression-free survival benefit from RVd plus ASCT than did those with del(17p), Dr. Richardson said. In addition, RVd plus ASCT yielded less progression-free survival benefit for Black participants, individuals with a body mass index greater than 25 kg/m2, and those with a higher disease stage.
The DETERMINATION trial was conducted in parallel with the French IFM 2009 study.3 The two had the same design, but DETERMINATION evaluated continuous lenalidomide maintenance, whereas IFM 2009 limited lenalidomide maintenance to 1 year. IFM 2009 demonstrated significantly superior progression-free survival with early transplant, and after almost 8 years of median follow-up, overall survival also remained similar but with almost 80% of patients having undergone delayed ASCT, in contrast to the 28% reported in DETERMINATION.4
The much longer progression-free survival for both arms in the DETERMINATION study vs IFM 2009 (which was 47 months with early transplant vs 35 months without) “suggests there is clearly a benefit to continuous maintenance,” Dr. Richardson said. “This comparison was preplanned, and the difference seen in favor of the continuous approach was striking. Given the benefit seen in other phase III studies, we are able to confirm that the use of lenalidomide maintenance until disease progression is now a standard of care.”
About DETERMINATION
The phase III DETERMINATION (Delayed vs Early Transplant With Revlimid Maintenance and Antimyeloma Triple Therapy) trial sought to determine whether first-line ASCT enhances the efficacy of triplet induction therapy or whether it can be kept in reserve for selected patients. The study’s 722 adults (aged 18–65 years) with symptomatic newly diagnosed myeloma received one cycle of RVd and then were randomly assigned to receive two additional RVd cycles plus stem cell mobilization and either five more RVd cycles (the RVd-alone group) or high-dose melphalan plus ASCT followed by two additional RVd cycles (the transplantation group).
For maintenance, both groups received daily lenalidomide (10 mg/d in months 1–3 and thereafter 15 mg/d) until disease progression. The median duration of maintenance was 41.5 months after RVd plus ASCT and 36.4 months after RVd alone. The primary endpoint was progression-free survival.
Notably, approximately 19% of trial participants were Black, which is apparently the highest representation of this subset of patients in any phase III trial in myeloma. “That was incredibly gratifying to see,” Dr. Richardson added.
MRD Negativity
MRD negativity is increasingly recognized as important for long-term outcomes in myeloma. It was more likely to be achieved for patients in the transplantation group, despite similar rates of complete responses between the two groups. Notably, treatment assignment was not found to be important for this group, as they had favorable 5-year progression-free survival regardless of treatment assignment: 53.5% with RVd plus ASCT and 59.2% with RVd alone (HR = 0.91; unadjusted 95% confidence interval [CI] = 0.46–1.79). For MRD-positive patients, RVd plus ASCT improved progression-free survival by 67% (HR = 1.67; unadjusted 95% CI = 0.98–2.85).
The finding is further evidence that sustained MRD negativity is a potentially valuable clinical endpoint. Treatment adaptation based on MRD status could be an alternative to the standard use of ASCT as well as maintenance until disease progression, according to the investigators.
More Toxicity
The advantages of the transplant arm came with the cost of significantly more grade ≥ 3 hematologic adverse events: 89.9% vs 60.5% (P < .001). Of note, after ASCT, 10 patients developed myelodysplastic syndrome and/or acute myeloid leukemia, compared with none in the RVd-alone arm (P = .002). Patients in the ASCT arm also had reductions from baseline in their quality of life, around the time of transplantation, but these decreases were reported to be transient and improved over time.
Recent trials and exploratory studies are “providing clues” that even newer approaches, such as quadruplet regimens that include monoclonal antibodies such as daratumumab or isatuximab have shown great promise and may produce even more “transformative” improvements than what has been shown in the DETERMINATION trial, Dr. Richardson noted.
DISCLOSURE: Dr. Richardson has served as a consultant or advisor to BMS/Celgene, Janssen, Karyopharm Therapeutics, Oncopeptides, Sanofi, SecuraBio and Takeda.
REFERENCES
1. Richardson PG, Jacobus SJ, Weller E, et al: Lenalidomide, bortezomib, and dexamethasone ± autologous stem cell transplantation and lenalidomide maintenance to progression for nearly diagnosed multiple myeloma: The phase 3 DETERMINATION trial. 2022 ASCO Annual Meeting. Abstract LBA4. Presented June 5, 2022.
2. Richardson PG, Jacobus SJ, Weller EA, et al: Triplet therapy, transplantation, and maintenance until progression in myeloma. N Engl J Med. June 5, 2022 (early release online).
3. Attal M, Lauwers-Cances V, Hulin C, et al: Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med 376:1311-1320, 2017.
4. Perrot A, Lauwers-Cances V, Cazaubiel T, et al: Early versus late autologous stem cell transplant in newly diagnosed multiple myeloma: Long-term follow-up analysis of the IFM 2009 trial. 2020 ASH Annual Meeting & Exposition. Abstract 143. Presented December 5, 2020.

