A first-in-human study presented at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) 2020 Annual Meeting and published by Zhang et al in the Journal of Nuclear Medicine has demonstrated the safety and favorable pharmacokinetic and dosimetry profile of Cu-64 EBRGD, a new, relatively long-lived positron-emission tomography (PET) tracer, in patients with glioblastoma. The radiotracer proved to be a superior high-contrast imaging diagnostic in patients, visualizing tumors that express low or moderate levels of αvβ3 integrin with high sensitivity.
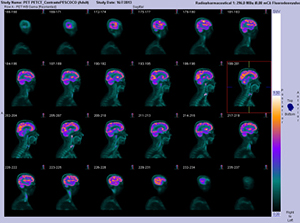
Photo credit: Getty
More About Cu-64 EBRGD
The Cu-64 EBRGD radiotracer presented in this study has several unique qualities. The peptide sequence Arg-Gly-Asp (RGD) specifically targets the cell surface receptor αvβ3 integrin, which is overexpressed in glioblastoma. To slow clearance, Evans Blue (EB) dye, which reversibly binds to circulating albumin, is bound to RGD, significantly enhancing target accumulation and retention. The addition of the Cu-64 label to EBRGD provides persistent, high-contrast diagnostic images in patients with glioblastoma patient.
Small First-in-Human Study
This first-in-human, first-in-class study included three healthy volunteers who underwent whole-body Cu-64 EBRGD PET/computed tomography (CT). Safety data—including vital signs, physical examination, electrocardiography, laboratory parameters, and adverse events—were collected after 1 day and after 1 week. Regions of interest were drawn, time-activity curves were obtained, and dosimetry was calculated. Two patients with recurrent glioblastoma also underwent Cu-64 EBRGD PET/CT. Seven sets of brain PET and PET/CT scans were obtained over 2 consecutive days. Tumor-to-background ratios were calculated for the target tumor lesion and normal brain tissue. One week after radiotracer administration, patients underwent surgical treatment and immunohistochemical staining of tumor samples was performed.
Results
Cu-64 EBRGD was well tolerated in patients with no adverse symptoms immediately or up to 1 week after administration. The mean effective dose of Cu-64 EBRGD was very similar to the effective dose of an F-18 fluorodeoxyglucose scan. Injection of Cu-64 EBRGD to the patients with recurrent glioblastoma showed high accumulation at the tumor with continuously increased tumor-to-background contrast over time. Postoperative pathology revealed World Health Organization grade IV glioblastoma, and immunohistochemical staining showed moderate expression of the αvß3 integrin.
“In this study, we have demonstrated a potential radiotheranostic agent that is safe, sensitive, and highly selective in humans, which infers a future diagnostic tool and breakthrough targeted radiotherapy for [patients with] glioblastoma,” said first study author Jingjing Zhang, MD, PhD, of Peking Union Medical College Hospital in Beijing, in a statement. “We believe this innovative use of Cu-64 EBRGD will significantly improve therapeutic efficacy and patient outcomes.”
Disclosure: For full disclosures of the study authors, visit jnm.snmjournals.org.

