Based on the phase III CLL17 trial, a fixed duration of targeted treatment demonstrated noninferiority to continuous treatment with respect to progression-free survival in previously untreated patients with chronic lymphocytic leukemia (CLL). The findings were presented at the Plenary Session of the 2025 American Society of Hematology (ASH) Annual Meeting & Exposition and simultaneously published in TheNew England Journal of Medicine.1,2
“This is the first phase III trial comparing the main paradigms of continuous vs fixed-duration targeted therapy in CLL. Early findings indicate that fixed-duration treatment with venetoclax–obinutuzumab or venetoclax–ibrutinib is noninferior to continuous treatment with ibrutinib and may therefore represent the preferred treatment option for patients with previously untreated CLL,” said Othman Al-Sawaf, MD, PhD, Hematologist and Medical Oncologist at the University Hospital Cologne in Germany.

Othman Al-Sawaf, MD, PhD
At a median observation time of 34.2 months, the 3-year progression-free survival rate was 81.1% in the venetoclax–obinutuzumab arm, 79.4% in the venetoclax–ibrutinib arm, and 81.1% in the ibrutinib arm. Compared to ibrutinib, the hazard ratios (HRs) were 0.87 (type I error–adjusted confidence interval [CI] = 0.54–1.41) and 0.84 (type I error–adjusted CI = 0.53–1.32), respectively; Dr. Al-Sawaf noted the upper limit of each adjusted confidence interval fell below the predefined noninferiority margin and thus provided early evidence of noninferiority.
Phase III CLL17 Trial
As Dr. Al-Sawaf explained, current treatment strategies for CLL follow two seminal paradigms: continuous treatment with a Bruton’s tyrosine kinase (BTK) inhibitor until disease progression, aimed at disease control; or fixed-duration regimens combining the BCL2 inhibitor venetoclax with a CD20 antibody or a BTK inhibitor, typically administered for 1 year, with the goal of achieving a deep remission and allowing a treatment-free interval. The optimal approach has not been established.
To compare these approaches, the investigators conducted a prospective, three-arm trial in patients with previously untreated CLL: (1) continuous ibrutinib monotherapy, (2) fixed-duration venetoclax–obinutuzumab, and (3) fixed-duration venetoclax–ibrutinib. Ibrutinib was given continuously until intolerance or disease progression. The venetoclax–obinutuzumab regimen consisted of 6 cycles (28 days each) of venetoclax plus obinutuzumab, followed by 6 additional cycles of venetoclax monotherapy; the venetoclax–ibrutinib regimen was initiated with a 3-cycle ibrutinib lead-in, followed by 12 cycles of venetoclax plus ibrutinib.
The international randomized phase III CLL17 study enrolled 909 treatment-naive patients (median age: 66 years), of whom 7.6% had del(17p) and/or TP53 mutation, 56.5% had unmutated IGHV, and 19.2% had complex karyotypes (≥ 3 aberrations). A total of 53.8% and 6.5% of patients were classified into a high- or very high–risk group, respectively, based on the CLL International Prognostic Index.
The study was designed to test the noninferiority of venetoclax–obinutuzumab and venetoclax–ibrutinib vs ibrutinib alone. The primary endpoint was investigator-assessed progression-free survival. A reduction in 3-year progression-free survival of less than or equal to 8% was deemed not clinically meaningful (noninferiority HR margin of 1.608). The interim analysis was performed when 65% of the progression-free survival events had occurred.
Noninferiority Demonstrated
“At this point, we can confirm noninferiority of progression-free survival. And as for overall survival, at 3 years we saw very favorable outcomes, with over 90% overall survival rates in all three arms, again confirming—with limited follow-up—that we have equal long-term outcomes,” Dr. Al-Sawaf said, though he acknowledged the occurrence of several fatal infections, particularly with venetoclax–obinutuzumab (22, of which 12 were COVID-19–related).
The 3-year overall survival rates were 91.5% with venetoclax–obinutuzumab, 96.0% with venetoclax–ibrutinib, and 95.7% with ibrutinib. At final staging, the objective response rates were 84.2%, 88.5%, and 86.0%, respectively, with complete responses achieved by 51.5%, 46.2%, and 8.3%. Undetectable measurable residual disease (MRD; < 10-4) in the peripheral blood was achieved in 73.3% of patients treated with venetoclax–obinutuzumab and 47.2% of those who received venetoclax–ibrutinib; no patient treated with continuous ibrutinib achieved this endpoint.
“Across the three arms, the response rates were more than 80%, but only the combination therapies were able to produce meaningful complete responses, which you do not see with single-agent therapy, and this also translated into the MRD findings,” he added. “You can see that with venetoclax–obinutuzumab, both in the peripheral blood and the bone marrow, the remissions were deepest, followed by venetoclax–ibrutinib, with no MRD negativity achieved with single-agent therapy. This translated to this progression-free survival benefit.”
A Closer Look at Subgroups
Dr. Al-Sawaf emphasized that the fixed-duration approach appeared to be noninferior in the general population of previously untreated patients with CLL (Table 1). But he said that, at this point, he will continue to take a more “cautious” approach in the small subset with TP53 mutations—8% of the study population—who may be destined for a more aggressive course.
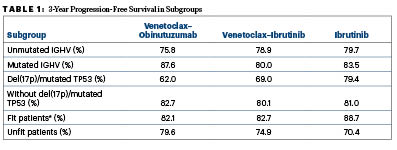
*Fitness defined as a Cumulative Illness Rating Scale score > 6 and/or glomerular filtration rate < 70 mL/min
“At this point, the study does not show substantial inferiority of fixed-duration treatment” in this subset, he said, but because the number of patients and their follow-up is limited, “I will put the 8% or so of patients with TP53 alterations on continuous treatment.” For other groups, the guidelines for German-speaking European countries have recently been revised to recommend that a limited duration of treatment be considered for all patients, even those with unmutated IGHV, he said during a press briefing.
Commenting on the outcomes in fit vs unfit patients, he suggested that coexisting conditions appeared to have less impact with fixed-duration therapy, largely because continuous BTK inhibition required more treatment interruptions, discontinuations, and dose modifications.
Safety Profile
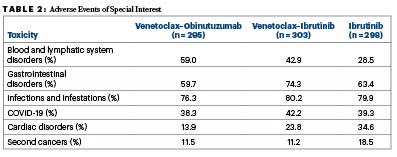
“Generally, we saw across the three groups that the safety profile is very similar to what has been observed before in the other studies where we had comparisons against chemotherapy, but we noticed some differences when we looked at the arms head-to-head,” Dr. Al-Sawaf said (Table 2).
“For instance, cytopenias (blood and lymphatic system disorders) were much more common with the combinations than with the single agent. Particularly with venetoclax–obinutuzumab, there was more neutropenia, but this occurred almost exclusively within 1 year of therapy and resolved after therapy. Other side effects such as cardiac disorders were more common with regimens containing ibrutinib, which is a known clinical challenge with BTK inhibitors. We also observed that infections and infestations affected almost 80% of patients across all three arms. Patients with CLL remain extremely vulnerable to infections, including COVID-19, but also bacterial pneumonia. Severe infections seemed to be particularly enriched with venetoclax–obinutuzumab, more so than with venetoclax–ibrutinib.”
“We conclude that fixed-duration treatments, both venetoclax–obinutuzumab and venetoclax–ibrutinib, administered over 1 year, are equally effective to continuous, indefinite therapy. We see more deep remissions with the combination therapies. But we also see an infection risk, which means our patients with CLL remain vulnerable to infections, so there is still an unmet need beyond the efficacy of treatment,” Dr. Al-Sawaf said. “We believe most patients with previously untreated CLL should be considered for fixed-duration treatments to enable these treatment-free intervals, but of course, longer follow-up will substantiate our findings further, especially regarding subgroups and also the long-term survival implications.”
DISCLOSURE: Dr. Al-Sawaf reported financial relationships with AbbVie, Janssen, Roche, BeOne Medicines (formerly BeiGene), Genmab, Lilly, and AstraZeneca.
REFERENCES
1. Al-Sawaf O, Stumpf J, Zhang C, et al: Fixed-duration versus continuous targeted treatment for previously untreated chronic lymphocytic leukemia: Results from the randomized CLL17 trial. 2025 ASH Annual Meeting & Exposition. Abstract 1. Presented December 6, 2025.
2. Al-Sawaf O, Stumpf J, Zhang C, et al: Fixed-duration versus continuous treatment for chronic lymphocytic leukemia. N Engl J Med. December 6, 2025 (early release online).
EXPERT POINT OF VIEW
For her thoughts on the CLL17 trial, The ASCO Post interviewed Susan O’Brien, MD, Professor of Medicine at the Chao Comprehensive Cancer Center at the University of California at Irvine.
Dr. O’Brien felt that the follow-up of CLL17 is too short to firmly conclude that fixed-duration Bruton’s tyrosine kinase (BTK) inhibitor plus venetoclax combinations are preferred over continuous BTK inhibition in previously untreated CLL. More appropriately, she said, the study “has not shown that continuous therapy is worse in any way except for the cardiac toxicity, which is not unexpected with continuous ibrutinib given over years.”

Susan O’Brien, MD
“The progression-free survival and response rates were essentially the same for the three arms, which doesn’t surprise me because they are all active therapies,” she said, adding that in the “long run” the combinations could have an edge, but that the 3-year mark is too early to tell. The lack of measurable residual disease negativity would be expected with single-agent ibrutinib; on this endpoint, venetoclax–obinutuzumab outperformed venetoclax–ibrutinib, she added.
Dr. O’Brien acknowledged that a fixed-duration approach has been her preference for most patients, with the main exception being those with TP53 mutations. The data from CLL17 appear to support this; in this subset, which accounted for 8% of the patients, she said, “The question is whether these patients need a BTK inhibitor or whether 1 year of treatment is not enough therapy. I don’t think we know, but since venetoclax–ibrutinib looked better than venetoclax–obinutuzumab in patients with 17p deletions, this suggests to me that they do need the BTK inhibitor.”
Tolerability is important in selecting treatment, she added. “One difference you do see among the arms in the study, which is not surprising, is in the incidence of cardiac events, where you see the most with continuous ibrutinib. You would expect this because venetoclax does not carry this risk. The second most common was with venetoclax–ibrutinib, but with [this regimen], you are only giving the ibrutinib for 1 year, not continuously. With venetoclax–obinutuzumab, you had more infection, mainly attributed to the obinutuzumab being immunosuppressive.”
Dr. O’Brien prescribes the better-tolerated second-generation BTK inhibitors instead of ibrutinib and believes that the findings of CLL17 would generally apply to those agents as well. Her approach aligns with the field in general, which—at least in the U.S.—is moving toward second-generation BTK inhibitors plus venetoclax as the preferred fixed-duration regimen.
DISCLOSURE: Dr. O’Brien has served as a consultant for AbbVie, AstraZeneca, Autolus, BeOne Medicines (formerly BeiGene), Bristol Myers Squibb, Eli Lilly, Epizyme, Janssen Oncology, Johnson & Johnson, and Pharmacyclics; has received research support from Alliance, Caribou Biosciences, Nurix Therapeutics, and Regeneron; and has served on a scientific advisory board for Sumitomo.

