For patients who develop severe diarrhea or colitis while receiving checkpoint inhibitors, the immunosuppressive agent vedolizumab is preferred over infliximab, according to an expert on the topic from The University of Texas MD Anderson Cancer Center. A retrospective comparison of outcomes with the two drugs was presented during the virtual edition of the American College of Gastroenterology (ACG) 2020 Annual Scientific Meeting by Yinghong “Mimi” Wang, MD, PhD, Director of Medication-Induced Colitis and Enteritis, Director of Fecal Microbiota Transplantation, and Associate Professor in the Department of Gastroenterology, Hepatology & Nutrition at MD Anderson.1 The study’s first author is Fangwen Zou, MD, also of MD Anderson.

“For colitis remission, the two agents are equally effective. However, if we’re talking about safety…, vedolizumab is a much safer medication [than infliximab] to use long term.”— Yinghong “Mimi” Wang, MD, PhD
Tweet this quote
“Compared with infliximab, long-term use of vedolizumab had equal efficacy in achieving clinical remission, better colitis disease course, lower colitis recurrence rates, less cancer progression at the last follow-up and better overall survival at 40 months,” Dr. Wang reported.
“For colitis remission, the two agents are equally effective. However, if we’re talking about safety—because we are dealing with a cancer population—based on the data, vedolizumab is a much safer medication to use long term,” she concluded.
Brooks D. Cash, MD, Chief of Gastroenterology, Hepatology & Nutrition at McGovern Medical School at UTHealth in Houston, commented on the importance of this analysis. “Gastroenterologists and oncologists alike should be aware of these data and consider them in their management of patients with checkpoint inhibitor–mediated colitis,” he said.
Infliximab Used Most Frequently
As Dr. Wang noted, immune-mediated diarrhea or colitis may limit treatment with checkpoint inhibitors. Steroids are the first-line treatment; patients who become refractory to steroids usually receive one of the two selective immunosuppressive therapies: infliximab or vedolizumab.
“There are multiple society guidelines but no clear evidence comparing the efficacy and safety of the two most frequently used nonsteroidal selective immunosuppressive agents for severe and refractory immunotherapy-mediated colitis,” she commented. She added that published data on efficacy come from the inflammatory bowel disease literature and not cancer populations.
Infliximab is more often the drug of choice of oncologists than vedolizumab, Dr. Wang said, because oncologists are more familiar with infliximab. “So far, oncology societies list infliximab as the first-line drug after failure of steroids and vedolizumab only after the failure of infliximab. That could be another limitation to vedolizumab’s use,” she said.
The administration of both drugs at MD Anderson presented the opportunity to compare their pros and cons. Dr. Wang and her team evaluated dosages, timing of administration, and effects on colitis course and cancer outcomes.
Retrospective Study of 150 Patients
Dr. Wang presented findings from a single-center retrospective study of 150 adults with cancer, mostly stage IV disease, treated with infliximab or vedolizumab after steroid treatment. The most common tumor types were genitourinary (37%), melanoma (35%), and lung (12%). For 23% of the patients, cancer progression was noted at the time of diagnosis of diarrhea or colitis.
Infliximab was given to 71 patients (47%), vedolizumab was given to 61 patients (41%), and combination of both agents (in sequential order) were given to 18 patients (12%). The type of checkpoint inhibitor was an anti–PD-1/L1 agent (49%), anti–CTLA-4 agent ipilimumab (18%), or a combination of the two (33%). The median length of treatment with immunotherapy was 70 days.
The median duration of symptoms of diarrhea or colitis was 50 days. Grade 3 or 4 diarrhea was reported in 70%, and grade 3 or 4 colitis was observed in 49%. About half (53%) had nonulcerative inflammation, whereas 29% had mucosal ulcerations on endoscopy exam. The number of steroid treatment courses was one to two for 55% of patients and three or more for 45%.
Most Factors Favored Vedolizumab
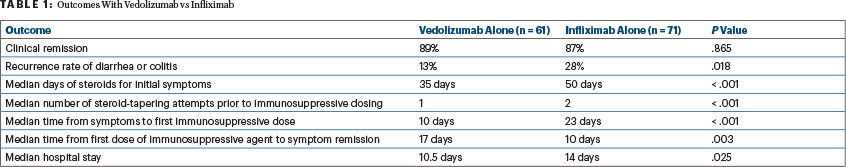
For numerous clinical outcomes, patients receiving vedolizu-mab for steroid-refractory diarrhea or colitis fared better (Table 1). Vedolizumab was introduced earlier after colitis onset, and those patients ultimately received fewer steroids and underwent fewer steroid-tapering attempts. The time to clinical response, however, was slightly longer with vedolizumab. In the vedolizumab group, hospital stays were shorter, and recurrence of diarrhea or colitis was less likely. Clinical remission of disease, however, was comparable between the treatment groups, Dr. Wang reported.
In a univariate analysis of recurrent symptoms, the sole factor shown to be protective was a higher dose of the selective immunosuppressive agent, especially patients receiving at least three doses had a significantly lower colitis recurrence (odds ratio = 0.66, P = .032).
KEY POINTS
- Researchers from MD Anderson Cancer Center compared outcomes between infliximab and vedolizumab in the treatment of steroid-refractory immunotherapy-related diarrhea or colitis.
- On multiple parameters, vedolizumab outperformed infliximab, though clinical remission rates were comparable.
- Patients receiving vedolizumab lived significantly longer than those receiving infliximab.
- Although currently, many oncologists are more likely to choose infliximab, they should become familiar with using vedolizumab.
Increased risk for recurrence, on the other hand, was associated with longer duration of checkpoint inhibitor treatment, longer duration of colitis symptoms, longer duration of steroid use, treatment with infliximab, and delayed introduction of the selective immunosuppressive agent. “After a colitis diagnosis, all these factors were associated with a higher risk,” Dr. Wang noted.
Cancer Progression and Overall Survival
Cancer progression was significantly associated with the presence of comorbidities, advanced cancer stage at immune checkpoint inhibitor initiation, longer duration of colitis symptoms, higher number of steroid-tapering attempts, and use of infliximab. Of note, recurrence of immune-mediated diarrhea or colitis was associated with a reduced risk of cancer progression.
The percentage of patients with disease progression at the time of colitis onset was 23% in the vedolizumab group and 28% in the infliximab group. At last follow-up, 34% of the vedolizumab group experienced disease progression (P = .245 vs baseline) compared with 53% of the infliximab group (P = .023 vs baseline). The 19% difference between the two treatment groups was also statistically significant (P = .028).
Finally, 60% of patients receiving vedolizumab were alive at 40 months compared with 45% of those receiving infliximab (P = .042). Researchers also found that a higher dose of selective immunosuppressive agent (eg, infliximab or vedolizumab; P = .026 vs a lower dose) and fewer steroid-tapering attempts (≤ 2 vs > 2) were associated with improved survival.
DISCLOSURE: Dr. Wang has served as a consultant or advisor to Tillotts Pharma AG. Dr. Cash reported no conflicts of interest.
REFERENCE


