In patients with hormone receptor–positive metastatic breast cancers with HER2-low or HER2-ultralow expression, treatment with the antibody-drug conjugate fam-trastuzumab deruxtecan-nxki (T-DXd) was found to be superior to chemotherapy after one or more lines of endocrine therapy. In DESTINY-Breast06, this approach significantly reduced the risk of disease progression by 38%, compared with chemotherapy, and patients with HER2-ultralow expression appeared to derive as much benefit as those in the HER2-low subset.1
“In DESTINY-Breast06, T-DXd demonstrated a statistically significant and clinically meaningful progression-free survival benefit vs chemotherapy in hormone receptor–positive, HER2-low metastatic breast cancer after one or more endocrine-based therapies, with consistent results in HER2-ultralow disease,” said Giuseppe Curigliano, MD, PhD, Professor of Oncology at the University of Milan, at a special session devoted to the results during the 2024 ASCO Annual Meeting.

Giuseppe Curigliano, MD, PhD

Julie R. Gralow, MD, FACP, FASCO
Why DESTINY-Breast06?
In the previous DESTINY-Breast04 trial, improvements were achieved with T-DXd in both progression-free survival (hazard ratio [HR] = 0.36) and overall survival (HR = 0.69) in HER2-low expressors after one to two prior lines of chemotherapy.2 In the current DESTINY-Breast06 trial, the drug’s efficacy was shown in an earlier setting—prior to chemotherapy—and was extended to the 17% of the study population with HER2-ultralow expression.
Breast cancer specialist Julie R. Gralow, MD, FACP, FASCO, Chief Medical Officer and Executive Vice President of ASCO, explained that DESTINY-Breast06 “moves T-DXd up one line” to be used prior to chemotherapy after disease progression on endocrine therapy. She continued: “What’s also new is the HER2-ultralow population, which we used to call HER2-0. We always knew that if you did ultrasensitive HER2 testing, there were receptors even in most of those tumors, but by immunohistochemistry, we didn’t see them.” With the results of this study, she added, “we are going to need a lot of discussion with our pathology colleagues for the reliability of just seeing a little bit of faint membrane staining vs none.”
About DESTINY-Breast06
DESTINY-Breast06 tested whether metastatic tumors with HER2-low or HER2-ultralow expression could benefit from the HER2-targeted antibody-drug conjugate. The study population was classified as follows:
- HER2-low: Immunohistochemistry (IHC) 2+ and in situ hybridization (ISH)-negative, characterized by weak to moderate complete membrane staining in more than10% of tumor cells, or IHC 1+, defined by faint, incomplete membrane staining in more than 10% of tumor cells
- HER2-ultralow: IHC 0, characterized by faint, incomplete membrane staining in up to 10% of tumor cells.
The trial included 866 patients with hormone receptor–positive metastatic breast cancer with HER2-low (n = 713) or HER2-ultralow (n = 153) expression. Patients were chemotherapy-naive in the metastatic setting but had received one of the following treatments:
- Two or more lines of endocrine therapy with or without targeted therapy for metastatic disease;
- One line of endocrine therapy for metastatic disease and disease progression within 6 months of starting first-line endocrine therapy plus a CDK4/6 inhibitor;
- One line of endocrine therapy for metastatic disease and recurrence within 24 months of starting adjuvant endocrine therapy.
Patients were randomly assigned to receive either T-DXd (5.4 mg/kg every 3 weeks) or standard-of-care chemotherapy—namely, capecitabine (60%), nab-paclitaxel (24%), or paclitaxel (16%). Previous therapy included two or more endocrine therapies in 85% and a CDK4/6 inhibitor in 90%. The primary endpoint was progression-free survival by blinded central review in the HER2-low population. Evaluation of the patients with HER2-ultralow expression was a key secondary endpoint.
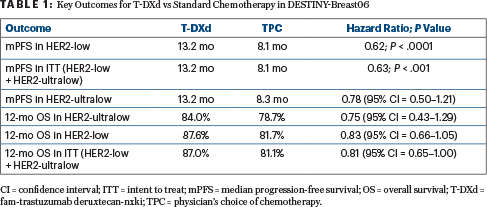
Improvement in Progression-Free Survival
After a median follow-up of 18.2 months, treatment with T-DXd resulted in a 5-month improvement in progression-free survival, translating to an almost 40% reduction in risk. Although overall survival was 40% mature, early outcomes were reported. T-DXd was heavily favored in all subgroups, according to the investigators. Confirmed objective response rates for patients receiving T-DXd were nearly twofold higher than for patients receiving chemotherapy (57% vs 31%), with consistency shown in the HER2-ultralow group.
Safety Profile
The rate of drug-related adverse events was generally higher with T-DXd than with chemotherapy. However, the median treatment duration was twice as long, 11 vs < 6 months, Dr. Curigliano pointed out. “There were no new safety signals, but interstitial lung disease remains a safety risk with T-DXd,” he added. Interstitial lung disease was reported in 49 patients (11.3%) treated with the agent, compared with 1 patient (0.2%) receiving chemotherapy. The vast majority of cases were grade 1 or 2, but there were three fatal events (0.7%). Decreases in left ventricular ejection fraction were more common with T-DXd, but no patients experienced cardiac failure while on this drug.
Expert Point of View

Ian E. Krop, MD, PhD
The DESTINY-Breast06 invited discussant, Ian E. Krop, MD, PhD, Professor of Medical Oncology at Yale School of Medicine, said that because of the positive results for T-DXd vs chemotherapy in a “clinically relevant population,” T-DXd can be considered a new standard of care in either the first or second line of therapy for hormone receptor–positive, HER2-low metastatic breast cancer.
The significant benefit for T-DXd in the previous DESTINY-Breast04 trial led to the drug’s approval in HER2-low breast cancer after one prior line of chemotherapy. Those trial results “also changed how we thought about HER2 expression in nonamplified cancers; that is, there is a continuum of expression,” Dr. Krop added. Data from DESTINY-Breast06 now “tell us we have to change again.… Even very low HER2 levels matter, and we can leverage this to improve treatments for our patients.”
What Next?
Now the questions become which patients need T-DXd, in what line of treatment, and how should they be identified? Although T-DXd improved outcomes over chemotherapy in the first-line setting, toxicity was worse (including more grade 3 events, cardiac toxicity, interstitial lung disease, and three deaths), and overall survival and quality-of-life data are lacking. “The bottom line is that it is an effective first option for patients after endocrine therapy, but it is unlikely that one approach will be optimal for all patients,” Dr. Krop concluded.
He continued: “Given the substantial overall survival benefit and high progression-free survival and response rate of T-DXd in the second line, who should receive T-DXd in the first vs second line?” Dr. Krop said he would consider first-line T-DXd for patients with symptomatic disease, a short interval after adjuvant chemotherapy, or preference for the drug. “However, the selection criteria may change as data evolve, particularly as overall survival matures,” he added.
In HER2-ultralow tumors, T-DXd does have “apparent efficacy,” Dr. Krop continued. “The similarity of the results to those in HER2-low suggests T-DXd is an effective first- or second-line option after endocrine therapy,” for the HER2–ultra-low cancers, he added, though acknowledging the data are not definitive in the second line. It is likely to be effective in that setting as well, he predicted, based on the DAISY trial.3 Whether T-DXd might work for cancers with no discernible HER2 expression is doubtful, according to Dr. Krop, “although the results are not definitive.”
Of note, DESTINY-Breast06 accentuates the need for more refined testing of HER2 expression to distinguish the HER2-low and HER2-ultralow cancers from the HER2-0 cancers. Multiple new assays are in development, but in the meantime, Dr. Krop commented, “pathologists are going to have to consider adding HER2-ultralow classification to our pathology reports for clinical use.”
DISCLOSURE: Dr. Curigliano reported financial relationships with Ellipses Pharma, AstraZeneca, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Celcuity, Daiichi Sankyo, Exact Sciences, Foundation Medicine, Gilead Sciences, GlaxoSmithKline, Guardant Health, Hengrui Therapeutics, Lilly, Menarini, Merck, Novartis, Pfizer, Roche/Genentech, Samsung, Seagen, and Veracyte. Dr. Gralow reported no conflicts of interest. Dr. Krop reported financial relationships with AstraZeneca, Daiichi Sankyo, Genentech/Roche, Merck, Novartis, and Seagen. His spouse is employed by and owns stock in PureTech.
REFERENCES
1. Curigliano G, Hu X, Dent RA, et al: Trastuzumab deruxtecan (T-DXd) vs physician’s choice of chemotherapy in patients with hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-low or HER2-ultralow metastatic breast cancer with prior endocrine therapy: Primary results from DESTINY-Breast06. 2024 ASCO Annual Meeting. Abstract LBA1000. Presented June 2, 2024.
2. Modi S, Jacot W, Iwata H, et al: 376O Trastuzumab deruxtecan (T-DXd) versus treatment of physician’s choice in patients with HER2-low unresectable and/or metastatic breast cancer: Updated survival results of the randomized, phase III DESTINY-Breast04 study. Ann Oncol 34(suppl 2):S334-S335, 2023.
3. Dieras V, Deluche E, Lusque A, et al: Trastuzumab deruxtecan for advanced breast cancer patients, regardless of HER2 status: A phase II study with biomarkers analysis (DAISY). 2021 San Antonio Breast Cancer Symposium. Abstract PD8-02. Presented December 9, 2021.

