Although head and neck cancers include multiple histologies and primary sites, squamous cell carcinomas (SCC) originating in the oropharynx, oral cavity, larynx, or hypopharynx are the most common. Today, we recognize different types of head and neck cancers, primarily those that are human papillomavirus (HPV)-positive and those that are HPV-negative. In the locally advanced curative-intent setting, a good prognosis has driven the study of de-escalation therapy in the former group, whereas a poor prognosis has led to escalation of therapy trials in the latter. Additionally, there is recurrent and/or metastatic head and neck SCC (HNSCC), where all systemic therapy is palliative, regardless of HPV status, and there continues to be a great need for improvement in outcomes for all patients. Important progress has been made recently, driving the field in new directions.

In the field of head and neck cancer, we strive for the next big jump in the recurrent and/or metastatic setting, like the one we achieved with anti–PD-1 monoclonal antibodies.— Dan P. Zandberg, MD
Tweet this quote
HPV-Positive Oropharyngeal SCC
Over the past 3 decades, it has become apparent that HPV causes SCC in the oropharynx, with a continued rise in incidence of these cancers over time. HPV-positive oropharyngeal cancer has a significantly better prognosis, with clinical risk stratification by smoking status and stage used to identify patients with low-risk cancer and intermediate-risk cancer.
Patients with intermediate-risk HPV-positive SCC are those with more than a 10 pack-year smoking history (where a pack-year is defined as the number of packs of cigarettes smoked per day multiplied by the number of years the person has smoked) and/or T4 or N3 disease by AJCC 8th edition staging.1,2 For patients with low-risk HPV-positive disease, the question becomes: Can we de-escalate our curative-intent therapy both to preserve the good prognosis and reduce toxicity, especially long-term toxicity, given these patients are often younger and without significant comorbidity? Although many de-escalation approaches are currently utilized, the most common involve reducing the radiation dosage and/or radiation field and/or omitting chemotherapy or replacing cisplatin with another systemic therapy. These approaches are being evaluated both as part of definitive nonsurgical therapy and also as adjuvant therapy after transoral robotic surgery (also known as TORS).
Presented during the 2020 ASCO Virtual Scientific Program, ECOG-ACRIN 3311 evaluated adjuvant strategies after transoral robotic surgery for patients with HPV-positive oropharyngeal cancer (Table 1).3 The primary comparison was in patients with pathologic intermediate-risk factors (close margins, < 1 mm extranodal extension, 2–4 lymph nodes, and/or perineural invasion/lymphovascular invasion) who were randomly assigned to adjuvant radiotherapy to a total dose of 50 Gy vs 60 Gy. These treatments resulted in a 2-year progression-free survival of 95.0% and 95.9%, respectively, with the study meeting its primary endpoint based on the upper bound of the confidence interval. Quality-of-life data will be important but have not been reported to date. Of note, this trial proved the feasibility of conducting a large, randomized transoral robotic surgery–based de-escalation trial and established adjuvant radiation therapy of 50 Gy as a promising alternative to standard-of-care treatment for HPV-positive oropharyngeal cancer with intermediate-risk pathologic features.3 This trial did not address de-escalation of therapy for patients with extranodal extension of more than 1 mm and/or positive margins, and it is an open question as to whether radiation therapy alone or with immunotherapy would be sufficient in this subgroup.
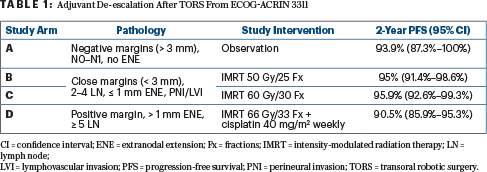
For nonsurgical therapy with definitive chemoradiation, the negative results of the randomized phase III trial RTOG 1016 taught us that cisplatin should not be replaced by cetuximab as a radiosensitizer. In addition, the phase II trial NRG-HN002 showed that although radiation therapy to 60 Gy plus weekly cisplatin appears acceptable for definitive treatment, 60-Gy radiotherapy alone does not.4,5 Currently, NRG-HN005 (ClinicalTrials.gov identifier NCT03952585), an important ongoing phase II/III trial, is randomly assigning patients to experimental arms of 60 Gy plus cisplatin and 60 Gy plus nivolumab or a control arm of 70 Gy plus cisplatin.
Although there are other de-escalation strategies being studied in this setting, and a full review of them is beyond the scope of this commentary, we will eventually need to consolidate approaches. Ideally, someday we will be able to compare the best nonsurgical de-escalation approaches to transoral robotic surgery followed by adjuvant de-escalated therapy, so we can counsel our patients as to the best approach for both efficacy and quality of life.
HPV-Negative HNSCC
The advances in management and high survival rates in patients with HPV-positive oropharyngeal SCC are welcome developments. By contrast, however, we haven’t been able to significantly improve outcomes in patients with locally advanced HPV-negative disease for decades. This has led to ongoing escalation trials, mostly combining chemoradiation with anti–PD-1/PD-L1 monoclonal antibodies in both the definitive and adjuvant settings.
During the 2020 European Society for Medical Oncology (ESMO) Virtual Congress, results of the phase III JAVELIN Head and Neck 100 trial were reported.6 This study compared chemoradiation with cisplatin plus concurrent and adjuvant avelumab for 1 year to chemoradiation plus placebo. Unfortunately, it was a negative trial, showing no difference in progression-free or overall survival. Although this was disappointing, we are awaiting the results of two additional phase III definitive chemoradiation trials with pembrolizumab (KEYNOTE-412; NCT03040999) and atezolizumab (IMvoke010; NCT03452137).
Preclinical data reveal that radiation has both immunogenic and suppressive properties, and our hope is that we can harness the former to synergize with immunotherapy to improve outcomes in these patients. However, we still have much to learn about how to optimize immunogenic dosing of radiation therapy, the effect of concurrent chemotherapy, and the best sequence of immunotherapy to make this happen.
We still have much to learn about what is the most immunogenic dosing of radiation therapy, the effect of concurrent chemotherapy, and the best sequence of immunotherapy.— Dan P. Zandberg, MD
Tweet this quote
Importantly, an ongoing phase II trial is directly comparing concurrent and adjuvant pembrolizumab plus chemoradiation with chemoradiation followed by pembrolizumab (NCT02777385). Notably, the atezolizumab is started after chemoradiation in IMvoke010, similar to the PACIFIC trial in non–small cell lung cancer (NSCLC). Additionally, patients with high PD-L1 expression were the only subgroup to benefit from the addition of avelumab in an exploratory analysis from JAVELIN, suggesting a biomarker-driven approach may be needed to select patients who may benefit from the addition of immunotherapy to chemoradiation. Also of interest is the incorporation of neoadjuvant immunotherapy, such as in the phase III KEYNOTE-689 trial currently accruing, given observed clinical and pathologic responses in prior “window of opportunity” trials that have leveraged this preoperative period for additional treatment.
For our patients with locally advanced, HPV-negative HNSCC, I hope that one or more of the ongoing phase III trials incorporating immunotherapy are positive. We will likely know over the next couple of years the results of IMvoke010 and KEYNOTE-412; consequently, we will learn whether we need to rethink our approach to improve outcomes for these patients. To that end, some early-phase chemoradiation trials are accruing, evaluating agents targeting DNA damage response pathways as radiosensitizers.
Recurrent and/or Metastatic HNSCC
Patients with recurrent and/or metastatic HNSCC face tremendous morbidity, and outcomes with systemic therapy remain poor for all patients regardless of HPV status. Progress, though, has been made in the past 5 years. In 2016, we saw the approval of nivolumab and pembrolizumab in the platinum-failure setting. More recently, in 2019, pembrolizumab was approved in the front-line setting as monotherapy or with platinum/fluorouracil (5-FU) chemotherapy based on PD-L1 expression as determined by combined positive score (CPS, ie, the number of PD-L1–staining cells divided by the total number of viable tumor cells, multiplied by 10). Nivolumab was the first drug to significantly improve overall survival in a randomized trial in patients with platinum-failure recurrent and/or metastatic HNSCC. And the new indication for pembrolizumab represents the first change in first-line standard of care since the EXTREME regimen (platinum/5-FU plus cetuximab) in 2011.
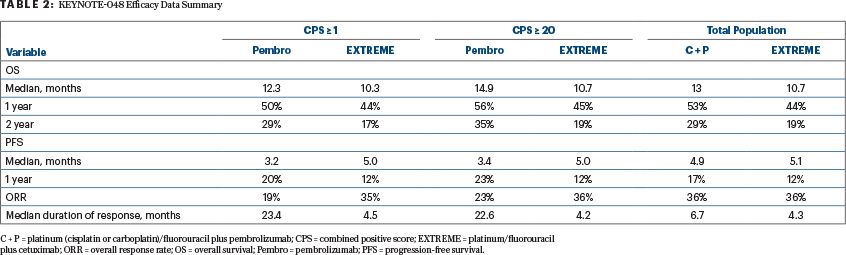
In the phase III KEYNOTE-048 trial, pembrolizumab monotherapy significantly improved overall survival for patients with either a PD-L1 CPS ≥ 1 or a CPS > 20, as did platinum/5-FU plus pembrolizumab for PD-L1 expressers and the total population, compared with the EXTREME regimen (Table 2). This led to U.S. Food and Drug Administration approval of pembrolizumab plus platinum/5-FU for all patients with recurrent and/or metastatic HNSCC and pembrolizumab monotherapy for patients with a PD-L1 CPS ≥ 1.7 Two other front-line phase III trials have already accrued: CheckMate 651 and KESTREL. CheckMate 651 is evaluating combination nivolumab plus ipilimumab, and KESTREL is assessing durvalumab with or without tremelimumab, both compared with the EXTREME regimen. While we await the results, KESTREL was reported to have failed to meet its primary endpoint of overall survival.
The success of KEYNOTE-048 has led to numerous new clinical trials in the front-line setting combining other agents with pembrolizumab monotherapy or platinum/5-FU plus pembrolizumab. Studies focused on PD-L1 expressers mostly sought to add another immunotherapy or targeted therapy to enhance efficacy in this space without the toxicity of cytotoxic chemotherapy, such as ongoing phase II/III trials with pembrolizumab in combination with lenvatinib and an inducible T-cell costimulatory agonist. Cetuximab plus an anti–PD-1 monoclonal antibody has also shown early promise. As more first-line combinations transition to phase III trials, it will be important to power trial comparisons by PD-L1 CPS range (1–19 and > 20) to best define how the combination can impact everyday practice.
With all the focus on PD-L1 expressers, we must not forget the 15% of patients with a PD-L1 CPS < 1; benefit in the “total population” from KEYNOTE-048 does not mean benefit in the population with PD-L1–negative disease. Subgroup analysis suggests no benefit to the addition of pembrolizumab for patients who are PD-L1–negative,8 but patient numbers are so small in this setting that firm conclusions cannot be made, and platinum/5-FU plus pembrolizumab is still the standard of care. Additionally, it is clear in HNSCC (and other solid tumors like NSCLC) that the addition of an anti–PD-1 agent to chemotherapy can improve responses, but the question remains whether we can achieve true synergy—ie, where the combination effect is greater than the probability of two agents acting independently.
Toward this end, we must gain a better understanding of the impact of chemotherapy on the tumor immune microenvironment in an effort to determine the best sequencing and type of chemotherapy to use. Ultimately, if we can find a way to capture both an increased response driven by chemotherapy and duration of response from anti–PD-1 drugs for all responders, it would be very meaningful to the field.
The success of front-line anti–PD-1 monoclonal antibody therapy has created an even greater need for better therapeutics for patients who experience disease progression on immunotherapy. Many phase I studies with novel combination immunotherapy are ongoing, with expansion cohorts in HNSCC. Ongoing earlier-phase trials include agents injected directly into tumor such as STING and TLR9 agonists. Although ideally an intratumoral injection would also boost a systemic response, significant regression of the injected lesions in the head and neck alone could significantly reduce morbidity. Promising activity with monalizumab, which targets NKG2A receptors, in combination with cetuximab has led to an ongoing phase III trial in the platinum- and immunotherapy-failure setting. Additionally, molecularly targeted therapy is making headway with both paclitaxel in combination with the PI3K inhibitor buparlisib and tipifarnib for HRAS-mutated HNSCC in later-stage trials.
It will be important to power trial comparisons by PD-L1 CPS range (1–19 and > 20) to best define how the combination can impact everyday practice.— Dan P. Zandberg, MD
Tweet this quote
What’s Next?
In the field of head and neck cancer, we strive for the next big jump in the recurrent and/or metastatic setting, like the one we achieved with anti–PD-1 monoclonal antibodies. Although I hope there will be a combination that provides durable benefit in the majority of patients, more realistically we will have to personalize the approach based on prospective tumor microenvironment analysis, harnessing the right tools for each individual from a large and growing toolbox, to take the next leap for these patients. In order to get there, we need to start testing these tumor microenvironment–driven strategies prospectively in trials, where clinical equipoise allows. We have started that approach with combination immunotherapy at the UPMC Hillman Cancer Center (NCT04326257).
Concluding Remarks
Our patients with HNSCC face unique morbidity, both from their disease and from the treatments themselves. Important work is being done in the locally advanced, curative-intent setting to find the best de-escalation strategy to reduce morbidity for patients with HPV-positive oropharyngeal cancer while preserving a good prognosis, as well as an escalation strategy for patients with HPV-negative disease to improve outcomes. The field continues to make strides in the recurrent and metastatic settings, where better therapeutics are still needed.
Although there have been some setbacks, there has also been great progress recently. Overall, it is an exciting time in the field of head and neck cancers, and researchers are poised to continue to move the needle forward in the coming years.
DISCLOSURE: Dr. Zandberg has received institutional research funding from Aduro Biotech, Astellas Pharma, AstraZeneca, Bristol Myers Squibb, Checkmate Pharmaceuticals, EMD Serono, Exelixis, GlaxoSmithKline, MacroGenics, Merck, and Regeneron; and has served on an advisory board for Blueprint Medicines.
REFERENCES
1. Ang KK, Harris J, Wheeler R, et al: Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24-35, 2010.
2. Huang SH, Xu W, Waldron J, et al: Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J Clin Oncol 33:836-845, 2015.
3. Ferris RL, Flamand Y, Weinstein GS, et al: Transoral robotic surgical resection followed by randomization to low- or standard-dose IMRT in resectable p16+ locally advanced oropharynx cancer: A trial of the ECOG-ACRIN Cancer Research Group (E3311). 2020 ASCO Virtual Scientific Program. Abstract 6500.
4. Gillison ML, Trotti AM, Harris J, et al: Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 393:40-50, 2019.
5. Yom SS, Torres-Saavedra P, Caudell JJ, et al: NRG-HN002: A randomized phase II trial for patients with p16-positive, non-smoking-associated, locoregionally advanced oropharyngeal cancer. Int J Radiat Oncol Biol Phys 105:684-685, 2019.
6. Cohen EE, Ferris RL, Psyrri A, et al: Primary results of the phase III JAVELIN Head & Neck 100 trial: Avelumab plus chemoradiotherapy (CRT) followed by avelumab maintenance vs CRT in patients with locally advanced squamous cell carcinoma of the head and neck. Ann Oncol 31(suppl 4):S658, 2020.
7. Burtness B, Harrington KJ, Greil R, et al: Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 394:1915-1928, 2019.
8. Burtness B, Rischin D, Greil R, et al: Abstract LB-258: Efficacy of first-line pembrolizumab by PD-L1 combined positive score < 1, 1-19, and ≥ 20 in recurrent and/or metastatic head and neck squamous cell carcinoma: KEYNOTE-048 subgroup analysis. Cancer Res 80(suppl):LB-258, 2020.
Dr. Zandberg is Associate Professor of Medicine and a medical oncologist, specializing in the treatment of head/neck and thyroid cancers. He is Director of the Head and Neck and Thyroid Cancer disease sections at the UPMC Hillman Cancer Center, Pittsburgh.

