Newly diagnosed patients with multiple myeloma deemed at high risk for disease progression may achieve sustained measurable residual disease (MRD) negativity with newer regimens and transplantation, and this may translate into longer progression-free survival. That’s the key take-away message from two studies presented at the 2021 ASCO Annual Meeting: the OPTIMUM/MUKnine trial from the United Kingdom,1 which focused on ultra-high–risk patients receiving a daratumumab-based regimen, and the FORTE trial from Italy,2 which evaluated a carfilzomib-containing regimen in a high-risk cohort.

Martin F. Kaiser, MD

Francesca Gay, MD, PhD
The results of OPTIMUM/MUKnine were presented by Martin F. Kaiser, MD, of the Institute for Cancer Research in London, and those of FORTE were described by Francesca Gay, MD, PhD, Associate Professor of Hematology at the University of Turin in Italy, who presented these findings on behalf of the GIMEMA European Myeloma Network.
OPTIMUM/MUKnine: High Rates of MRD Negativity
The OPTIMUM/MUKnine trial evaluated daratumumab, cyclophosphamide, bortezomib, lenalidomide, and dexamethasone (Dara-CVRd) induction, bortezomib-augmented high-dose melphalan, and autologous stem cell transplantation (ASCT). After transplantation, patients received daratumumab-based consolidation and maintenance therapies.
“To our knowledge, this is the first report on a trial for ultra-high–risk newly diagnosed myeloma and plasma cell leukemia investigating Dara-CVRd induction and augmented ASCT. Response rates were high in this difficult-to-treat patient population, with toxicity comparable to other induction regimens,” Dr. Kaiser said.
Dr. Kaiser reported the early protocol-defined endpoints from induction to day 100 after ASCT in 107 patients with ultra-high–risk newly diagnosed myeloma (at least two high-risk abnormalities by genetic and gene-expression profile) or plasma cell leukemia, enrolled at 39 hospitals. Double-hit genetic alterations were identified in 53%, the SKY92 risk signature was seen in 77%, and 31% had both these high-risk features. Of the 107 who received at least one dose of study drug, 89 ultimately were evaluable 100 to 120 days after transplantation.
“Dara-CVRd induction and bortezomib-augmented transplantation induced deep remissions in ultra-high–risk myeloma and primary plasma cell leukemia.”— Martin F. Kaiser, MD
Tweet this quote
These patient subsets have in common a median progression-free survival of less than 24 months with standard induction therapy and transplantation, making them a “biologically defined ultra-high–risk disease group” that might fare better with “modern combination therapy,” according to Dr. Kaiser.
OPTIMUM/MUKnine: Key Details
Patients received up to six cycles of Dara-CVRd induction, high-dose melphalan and ASCT augmented with bortezomib, followed by Dara-VRd consolidation for 18 cycles and Dara-R maintenance. Primary trial endpoints were MRD status after ASCT (reported here) and progression-free survival.
MRD assessment by flow cytometry (10-5 sensitivity) was available for 81% of patients after induction and 78% at day 100 after ASCT. Progression-free survival will be compared with matched ultra-high–risk controls from Myeloma XI and reported later. Median follow-up was 22 months.
“Dara-CVRd induction and bortezomib-augmented transplantation induced deep remissions in ultra-high–risk myeloma and primary plasma cell leukemia,” Dr. Kaiser reported (Table 1).
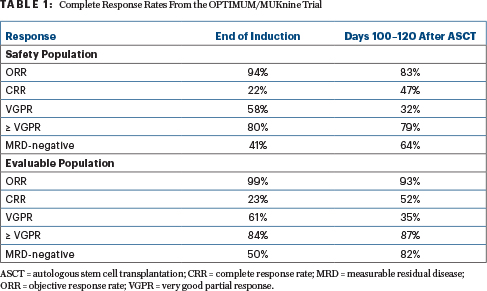
In the eight patients with plasma cell leukemia, response rates were 75%, including 25% with complete responses and 50% with at least a very good partial response.
Progression-free survival data are still maturing, but Dr. Kaiser indicated that some patients have relapsed early “despite this intensified modern therapy approach.” He believes this demonstrates “that risk in myeloma is quantitative, and there is an unmet need that persists in subgroups within the ultra-high–risk category.”
“Dara-CVRd induction and bortezomib-augmented transplantation induced deep remissions in ultra-high–risk myeloma and primary plasma cell leukemia,” Dr. Gay said.
FORTE: Details in High-Risk Patients
The FORTE study evaluated carfilzomib/lenalidomide/dexamethasone (KRd) induction/consolidation with ASCT, with further randomization to maintenance carfilzomib/lenalidomide (KR) or lenalidomide, in 474 newly diagnosed transplant-eligible patients up to age 65. Patients at high risk were defined as having one or more of these alterations: t(4;14), t(14;16), del(17p), gain(1q), and amp(1q). Patients with double-hit disease had at least two such alterations, and standard-risk patients had none.
“Achieving and maintaining MRD negativity in high-risk patients is a very important prognostic factor.”— Francesca Gay, MD, PhD
Tweet this quote
As previously reported,3 the study found improved progression-free survival with KRd and ASCT vs 12 cycles of KRd without ASCT (hazard ratio [HR] = 0.61; P = .0084) or carfilzomib/cyclophosphamide/dexamethasone (KCd) plus ASCT (HR = 0.55; P < .001). Maintenance with KR also significantly improved progression-free survival vs lenalidomide alone (HR = 0.64; P = .02294).
By risk group, in high-risk patients, KRd plus transplantation reduced the risk of disease progression by 40% (P = .04) vs 12 cycles of KRd (KRd12) and by 43% vs KCd plus transplantation (P = .01), with even a greater benefit seen in the double-hit group, which constituted 30% of the -population.
“The benefit of KRd and transplantation over KRd continuous treatment and KCd and transplantation was retained in standard-risk, high-risk, and double-hit patients,” commented Dr. Gay. The 4-year progression-free survival was around 80% for patients with standard-risk disease and approximately 60% for those with high-risk and double-hit disease.
FORTE is also evaluating progression-free survival and 1-year MRD negativity according to the presence of these high-risk chromosomal abnormalities, which was the focus of Dr. Gay’s presentation at the ASCO meeting.
Progression-Free Survival by Abnormality
Although the analysis of outcomes according to genomic abnormality may be limited by the small numbers, the study nevertheless showed a benefit with KRd plus transplantation over continuous KRd and KCd plus transplantation, especially for patients with del(17p) and those with gain(1q), Dr. Gay reported (Table 2).
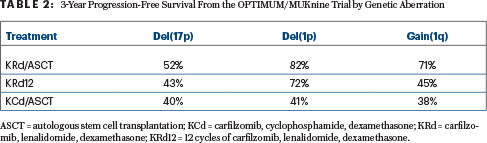

For patients with del(1p), progression-free survival was similar with KRd plus transplantation and continuous KRd—both being superior to KCd. Limited benefit was seen for patients with t(4;14), and no real benefit was reported for the amp(1q) subgroup—patients with the poorest outcomes overall.
MRD Negativity in High-Risk Patients
MRD negativity in high-risk patients mirrors the results shown in the main study. Premaintenance rates with KRd and transplantation were similar to those with continuous KRd and superior to MRD negativity rates achieved with KCd plus transplantation in patients with high-risk and double-hit disease. In high-risk patients, MRD negativity was observed in 59% after KRd plus ASCT, 62% after KRd12, and 47% after KCd plus ASCT. For patients with double-hit disease, these rates were 44%, 50%, and 39%, respectively.
“However, when you look at the sustained 1-year rates of MRD negativity, you see patients from the KRd-plus-ASCT arm have a significantly higher rate than those given KCd plus ASCT and compared with KRd continuous treatment. A difference was particularly evident between KRd plus transplantation and KRd12 in patients with double-hit disease,” Dr. Gay reported (Table 3).
“The progression-free survival of high-risk and double-hit patients getting to MRD negativity and maintaining this for at least 1 year is very good, with a 4-year progression-free survival of around 85%. Again, this highlights that achieving and maintaining MRD negativity in high-risk patients is a very important prognostic factor,” Dr. Gay said.
Benefit of Carfilzomib Maintenance
The benefit of carfilzomib maintenance was seen in all three risk groups. The 3-year progression-free survival after starting KR maintenance was 90% in the standard-risk group (HR = 0.40; P = .05), 69% in the high-risk group (HR = 0.60; P = .04), and 67% in the double-hit group (HR = 0.53; P = .1). The benefit of KR vs lenalidomide was retained in patients with del(17), del(1p), gain(1q), and t(t:14) but not in patients with amp(1q)—who, again, had the poorest outcomes in the study, Dr. Gay said.
DISCLOSURE: Dr. Kaiser has received honoraria from Amgen, Celgene, Janssen Oncology, and Takeda; has served as a consultant or advisor to AbbVie, Amgen, Bristol Myers Squibb, Celgene, Chugai Pharma, GSK, Janssen Oncology, Seattle Genetics, and Takeda; has received institutional research funding from Celgene; and has been reimbursed for travel, accommodations, or other expenses by Bristol Myers Squibb/Celgene, Janssen, and Takeda. Dr. Gay has received honoraria from AbbVie, Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, and Takeda; and has served as a consultant or advisor to AbbVie, Adaptive Biotechnologies, Amgen, Bluebird Bio, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Oncopeptides, Roche, and Takeda.
REFERENCES
1. Kaiser MF, Hall A, Walker K, et al: Depth of response and minimal residual disease status in ultra high-risk multiple myeloma and plasma cell leukemia treated with daratumumab, bortezomib, lenalidomide, cyclophosphamide and dexamethasone: Results of the UK optimum/MUKnine trial. 2021 ASCO Annual Meeting. Abstract 8001. Presented June 8, 2021.
2. Gay F, Mina R, Rota-Scalabrini D, et al: Carfilzomib-based induction/consolidation with or without autologous transplant followed by lenalidomide or carfilzomib-lenalidomide maintenance: Efficacy in high-risk patients. 2021 ASCO Annual Meeting. Abstract 8002. Presented June 8, 2021.
3. Gay F, Cerrato C, Petrucci MT, et al: Efficacy of carfilzomib, lenalidomide, dexamethasone with or without transplantation in newly diagnosed myeloma according to risk status: Results from the FORTE trial. 2019 ASCO Annual Meeting. Abstract 8002. Presented May 26, 2019.

