Outcomes in patients with triple-class–failure relapsed and refractory multiple myeloma who experience disease progression on immunomodulatory agents, proteasome inhibitors, and CD38 antibodies are dismal. Most recently, early results of three anti-B-cell maturation antigen (BCMA) chimeric antigen receptor (CAR)-modified T-cell therapies in highly refractory patient populations were reported.1-3 In this installment of the Hematology Expert Review, we provide an overview of these advancements.
Once more data are published, it would be interesting to carefully review and compare different anti-BCMA strategies—ie, CAR T-cell therapy, bispecific T-cell engager molecule (eg, AMG 420), and antibody-drug conjugates such as belantamab mafodotin—with regard to their safety, efficacy, and (overall) cost.
GUEST EDITORS

Syed Ali Abutalib, MD

Shaji K. Kumar, MD
KarMMa Trial: Idecabtagene Vicleucel
ABSTRACT 8503: Idecabtagene vicleucel (bb2121) in patients with relapsed and refractory multiple myeloma: Initial results from KarMMa study1 (ClinicalTrials.gov identifier NCT03361748)
Methods: Enrolled patients were refractory to at least three prior regimens (including immunomodulatory agents, proteasome inhibitors, and CD38 antibodies), and all patients were refractory to their last regimen. About 84% of the patients were triple-class refractory. After conventional lymphodepletion with cyclophosphamide (300 mg/m2) and fludarabine (30 mg/m2) for 3 days, patients received planned dose escalation from 150 × 106 (n = 4) → 300 × 106 (n = 70) → 450 (n = 54) × 106 CAR T cells.
Results: Of 140 patients who underwent leukapheresis, 128 received idecabtagene vicleucel. About 88% of the participants received bridging therapy between the period of T-cell collection and CAR T-cell infusion; 4% responded to it (one very good partial remission and four partial responses). The overall response rate was 73% (primary endpoint), and median progression-free survival was 8.8 months (95% confidence interval [CI] = 5.6–11.6 months); both outcomes improved with higher doses of CAR T cells. The median time to complete response was 2.8 months (range, 1–11.8 months). The duration of response was longer with the higher dose and in patients who achieved deeper responses.
Most common (any-grade) toxicities were cytopenias (97%; not dose-related) and cytokine-release syndrome (84%; Lee criteria).4 The frequency of cytokine-release syndrome increased with the higher dose of CAR T cells and developed in 107 patients (84%); most cases were grade 1 and 2, although 5 cases were grade 3, one case was grade 4, and one patient died of cytokine-release syndrome (300 × 106 dose). Tocilizumab was administered in 52% of patients with cytokine-release syndrome. Neurotoxicity was mostly low grade and similar across the three target doses; it was noted in 23 patients (18%); 4 cases were grade 3. Six patients died within 6 months of idecabtagene vicleucel infusion (Table 1).
Clinical Implications: Efficacy and safety data reported here are consistent with a prior published report5 and support a favorable idecabtagene vicleucel clinical benefit-risk profile across the target dose of 300 to 450 × 106 CAR T cells, with an overall dropout rate of approximately 8%.
A retrospective study,6 also presented during the ASCO20 Virtual Scientific Program, compared outcomes of the “real-world cohort” (n = 190) in patients who met KarMMa eligibility criteria (and received currently available therapies) and the KarMMa study cohort (n = 128). With a median follow-up of 11.3 months for the KarMMa study and 10.2 months for the “real-world cohort,” the rates of overall response, very good partial response, and progression-free survival were significantly (P < .0001) improved in patients on the KarMMa study vs patients in the “real-world cohort.” Although the median progression-free survival of 8.8 months may appear disappointing, however, these patients were heavily pretreated, with few (if any) alternative options.
CARTITUDE-1 Trial: JNJ-4528
ABSTRACT 8505: A phase Ib/II study of JNJ-4528 CAR T-cell therapy in relapsed and refractory multiple myeloma: Update of CARTITUDE-12 (NCT03548207)
Methods: A total of 35 patients were enrolled, 2 withdrew, and 4 died before infusion. The patients had received at least three prior regimens or were double-refractory to immunomodulatory agents and proteasome inhibitors and had received anti-CD38 antibody; 97% of patients were refractory to their last regimen. Nearly 86% were triple class–refractory. After conventional lymphodepletion with cyclophosphamide (300 mg/m2) and fludarabine (30 mg/m2) for 3 days, patients received JNJ-4528 at a median dose of 0.73 x106 (0.52–0.89 x 106) CAR-viable T cells/kg. Subjects have been treated with this construct using single-agent lymphodepletion with cyclophosphamide, with manageable toxicities (LEGEND-2 study); results were presented during the 2019 American Society of Hematology (ASH) Annual Meeting & Exposition.7
Results: In this trial, the most frequent adverse events were neutropenia (100%), cytokine-release syndrome (93%; Lee criteria4), and thrombocytopenia (93%). Grade ≥ 3 hematologic adverse events were neutropenia (100%), thrombocytopenia (69%), and leukopenia (66%). Cytokine-release syndrome was seen in 27 patients (93%); it was grade 1 to 2 in 25 patients, at least grade 3 in 2 patients, and 1 patient died of it. Neurotoxicity was seen in three patients; it was grade 1 to 2 in two patients and grade 3 in one patient. The overall response rate was 100%, with a 9-month progression-free survival of 86% (95% CI = 67%–95%; Table 1).
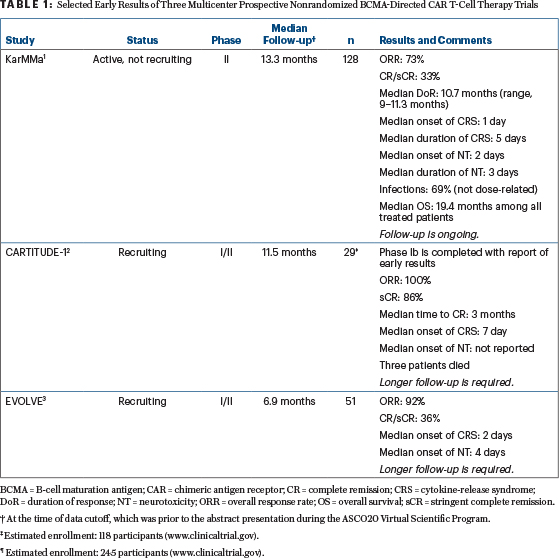
Clinical Implications: These early results are from the phase Ib cohort. The responses were early and durable at a low dose of CAR-viable T cells, but follow-up remains short. The JNJ-4528 CAR T-cell therapy has received Breakthrough Therapy designation; the phase II portion of the study is fully enrolled (CARTITUDE-2; NCT04133636), and phase III (CARTITUDE-4; NCT04181827) studies have been initiated.
EVOLVE Trial: Orvacabtagene Autoleucel
ABSTRACT 8504: Orvacabtagene autoleucel (JCARH125), a CAR T-cell therapy for patients with relapsed and refractory multiple myeloma: Update of EVOLVE study3 (NCT03430011)
Methods: Enrolled patients had received at least three prior regimens, including immunomodulatory agents, proteasome inhibitors, and anti-CD38 antibody, and all patients (n = 62) were refractory to their last regimen. About 94% of patients had prior autologous transplantation and were triple class–refractory. After conventional lymphodepletion with cyclophosphamide (300 mg/m2) and fludarabine (30 mg/m2) for 3 days, patients received orvacabtagene autoleucel at dose levels of 300 x 106 (n = 19), 450 x 106 (n = 19), and 600 × 106 (n = 24) CAR T cells. A total of 28 of 39 patients were also refractory to bridging therapy. Data on patients treated with 50 x 106 and 150 x 106 have been previously reported during the 2018 ASH Annual Meeting & Exposition,8 with overall response rates of 79% and 86%, respectively.
Results: Dose-limiting toxicities in this cohort were seen in two patients: grade 3 neurotoxicity for more than 7 days at 300 × 106 CAR T cells and grade 4 neutropenia for more than 28 days at 450 × 106 CAR T cells. Grade ≥ 3 anemia, neutropenia, and thrombocytopenia occurred in 21%, 55%, and 44% of patients, respectively. Grade ≥ 3 infections occurred in 13% of patients. Macrophage activation syndrome/hemophagocytic lymphohistiocytosis developed in three patients, and one died on day +51. Another patient died of cardiac arrest on day +53. After a median follow-up of 5.9 months, the overall response rate was 91%, with median progression-free survival not reached (Table 1).
Clinical Implications: At early follow-up, orvacabtagene autoleucel at all dose levels had a manageable safety profile, with rates of grade ≥ 3 cytokine-release syndrome and neurotoxicity of 2% and 4%, respectively, with compelling efficacy. Enrollment at the recommended dose of 600 × 106 CAR T cells is ongoing. Interestingly, the protocol was amended to include a phase IIa cohort whose disease progressed prior to BCMA-directed therapy. This is an important and unexplored clinical question, and the answer, most probably, will unveil clinically relevant information.
DISCLOSURE: Dr. Abutalib has served on the advisory board for AstraZeneca and Partner Therapeutics. Dr. Kumar has served as a consultant or advisor to Cellectar; has served in an institutional consulting or advisory role for AbbVie, Amgen, Bluebird Bio, Celgene, GeneCentrix, Genentech/Roche, Janssen Oncology, Kite Pharma, Merck, Oncopeptides, and Takeda; has received institutional research funding from AbbVie, Carsgen Therapeutics, Celgene, Janssen Oncology, Kite Pharma, MedImmune, Merck, Novartis, Roche/Genentech, Sanofi, Takeda, and TeneoBio.
REFERENCES
1. Munshi NC, Anderson Jr LD, Shah N, et al: Idecabtagene vicleucel (bb2121), a BCMA-targeted CAR T-cell therapy, in patients with relapsed and refractory multiple myeloma: Initial KarMMa results. ASCO20 Virtual Scientific Program. Abstract 8503.
2. Bardeja JG, Madduri D, Usmani SZ, et al: Update of CARTITUDE-1: A phase Ib/II study of JNJ-4528, a B-cell maturation antigen–directed CAR-T-cell therapy, in relapsed/refractory multiple myeloma. ASCO20 Virtual Scientific Program. Abstract 8505.
3. Mailankody S, Jakubowiak AJ, Htut M, et al: Orvacabtagene autoleucel, a B-cell maturation antigen–directed CAR T cell therapy for patients with relapsed/refractory multiple myeloma: Update of the phase 1/2 EVOLVE study (NCT03430011). ASCO20 Virtual Scientific Program. Abstract 8504.
4. Lee DW, Gardner R, Porter DL, et al: Current concepts in the diagnosis and management of cytokine release syndrome [published correction appears in Blood 126:1048, 2015. Dosage error in article text]. Blood 124:188-195, 2014.
5. Raje N, Berdeja J, Lin Y, et al: Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med 380:1726-1737, 2019.
6. Jagannath S, Lin Y, Goldschmidt H, et al: KarMMa-RW: A study of real-world treatment patterns in heavily pretreated patients with relapsed and refractory multiple myeloma and comparison of outcomes to KarMMa. ASCO20 Virtual Scientific Program. Abstract 8525.
7. Wang BY, Zhao WH, Liu J, et al: Long-term follow-up of a phase 1, first-in-human open-label study of LCAR-B38M, a structurally differentiated chimeric antigen receptor T cell therapy targeting B-cell maturation antigen, in patients with relapsed/refractory multiple myeloma. 2019 ASH Annual Meeting & Exposition. Abstract 579.
8. Mailankody S, Htut M, Lee KP, et al: JCARH125, anti-BCMA CAR T-cell therapy for relapsed/refractory multiple myeloma: Initial proof of concept results from a phase 1/2 multicenter study (EVOLVE). 2018 ASH Annual Meeting & Exposition. Abstract 957.

