Clinicians who treat multiple myeloma can anticipate a host of new treatments: melflufen, cereblon E3 ligase (CEL) modulators, antibody-drug conjugates, bispecific antibodies, and chimeric antigen receptor (CAR) T-cell therapies. Kenneth C. Anderson, MD, Director of the Jerome Lipper Multiple Myeloma Center at Dana-Farber Cancer Institute and the Kraft Family Professor of Medicine at Harvard Medical School, shared his enthusiasm over these novel treatment approaches in a presentation during the 2020 Debates and Didactics in Hematology and Oncology Virtual Conference, sponsored by Emory University Winship Cancer Institute.1
“In the future, we will be able to achieve minimal residual disease negativity with our targeted therapies, and restore host antimyeloma immunity with immunotherapy approaches,” Dr. Anderson said. “When we do both, we will achieve long-term disease-free survival and potential cure.”
Melflufen: A New Alkylating Agent
Melflufen is a first-in-class peptide-conjugated alkylating agent that targets aminopeptidases, which are abundant in myeloma. It is a melphalan prodrug that is rapidly taken up by myeloma cells and hydrolyzed to release an alkylator payload. “The idea is to get a better therapeutic index with this drug,” Dr. Anderson said.
On August 29, 2020, the U.S. Food and Drug Administration (FDA) granted Priority Review for melflufen in combination with dexamethasone for patients whose disease is refractory to at least one proteasome inhibitor, one immunomodulatory drug, and one anti-CD38 antibody (ie, triple-refractory). The submission is based on the results from the pivotal phase II study HORIZON, which involved 121 patients with relapsed or refractory disease, 93 of whom had triple-refractory disease. At the 2019 American Hematology Association (ASH) Annual Meeting & Exposition, Mateos et al reported the response rate to melflufen was 29% overall and 24% in triple-refractory patients.2 The median duration of response was 7.5 months, the median progression-free survival was 4.0 months, and the median overall survival was 11.3 months. Efficacy for melflufen was comparable in patients with extramedullary disease and in those with high-risk cytogenetics.

“In the future, we will be able to achieve minimal residual disease negativity with our targeted therapies and to restore host antimyeloma immunity with immunotherapy approaches.”— Kenneth C. Anderson, MD
Tweet this quote
Melflufen plus dexamethasone is also being compared with pomalidomide/dexamethasone in the phase III OCEAN trial of patients whose disease is refractory to lenalidomide.
CEL Modulators: Potent Immunomodulatory Drugs
Cereblon E3 ligase modulators are designed for rapid and maximal degradation of target proteins; they have profound immune-stimulatory effects and tumoricidal activity in myeloma cell lines. In fact, CEL modulators are the most potent immunomodulatory drugs yet to be used in myeloma, binding to cereblon (which mediates the action of immunomodulatory drugs) with a much higher affinity than pomalidomide, noted Dr. Anderson.
The oral CEL modulator CC-92480 was evaluated in a multicenter international phase I study reported by Richardson et al during the ASCO20 Virtual Scientific Program.3 The 76 heavily pretreated patients (half triple-refractory) received various doses of CC-92480 plus dexamethasone. At the recommended phase II dose—1 mg/d, 2 weeks on and 1 week off—the response rate was 55%, and the disease control rate was 100%.
“Even in triple-refractory patients, and in those resistant to pomalidomide, there were high extent and rate of response,” Dr. Anderson noted.
BCMA-Targeted Immunotherapy: Focus on Belantamab Mafodotin
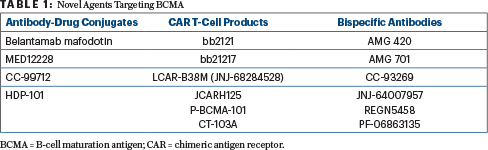
The B-cell maturation antigen (BCMA) on myeloma and plasma cells is being therapeutically targeted in several ways: with antibody-drug conjugates linked to a BCMA antibody, with BCMA--targeting bispecific T-cell engagers, and with a BCMA antibody linked to CAR T cells (Table 1). “This is a very, very active area of research,” Dr. Anderson revealed. Belantamab mafodotin and bb2121 are currently in phase III clinical trials. LCAR-B38M, JCARH125, P-BCMA-101, AMG-420, AMG-701, and REGN5458 are in phase II trials. Other such agents are in phase I or preclinical studies.
The antibody-drug conjugate belantamab mafodotin, which links a BCMA antibody to auristatin immunotoxin, has a dual mechanism of action in myeloma. The immunotoxin is delivered selectively to the BCMA-positive myeloma cells, and it is also internalized, resulting in direct apoptosis of myeloma cells.
The DREAMM-2 trial included 196 patients refractory to a proteasome inhibitor and an immunomodulatory drug as well as exposed to an anti-CD38 antibody (> 90% refractory to it); patients received 2.5 or 3.4 mg/kg of single-agent belantamab mafodotin.4 In an updated analysis performed at 13 months and presented during the ASCO20 Virtual Scientific Program,5 the overall response rate was 32% with 2.5 mg/kg and 35% with 3.4 mg/kg of belantamab mafodotin, which included a very good partial response or better in 58% and 66%, respectively.
The median duration of response was 11.0 months and 6.2 months, respectively; the median progression-free survival was 2.8 and 3.9 months; the median overall survival was 14.9 and 14.0 months, respectively; and the estimated 1-year survival was 58% in both dose cohorts. The dose of 2.5 mg/kg was chosen for further studies. “About one-third of patients responded, even though they had highly refractory disease,” Dr. Anderson commented, “and the extent of response was significant.”
Grade ≥ 3 keratopathy/microcystic-like epithelial changes were observed in 46% of patients in the lower-dose group and 42% of those in the higher-dose group. Although this caused dose delays in about one-quarter of patients, almost no patients discontinued therapy because of keratopathy/microcystic-like epithelial changes. “Visual changes resolved and did not have a significant long-term impact on quality of life,” noted Dr. Anderson.
On August 5, 2020, the FDA granted accelerated approval to belantamab mafodotin-blmf for adult patients with relapsed or refractory multiple myeloma who have received at least four prior therapies, including an anti-CD38 monoclonal antibody, a proteasome inhibitor, and an immunomodulatory agent.
Bispecific T-Cell Engagers
The BCMA-targeted bispecific T-cell engagers bind both to BCMA on multiple myeloma cells and to CD3 on T cells. “The concept with this off-the-shelf medication is to bring T cells to the -BCMA-positive myeloma cell and lyse them,” Dr. Anderson explained.
Many bispecific T-cell engagers are in clinical trials, including CC-93269, a human IgG1-based T-cell engager that binds to BCMA and CD3 epsilon in a 2+1 format. In a dose-escalation trial of 30 patients with relapsed or refractory disease, the 10-mg dose of CC-93269 induced responses in 89% of patients, including complete or stringent complete remission in 44%.6 Minimal residual disease negativity was achieved by 92% of responders. Although cytokine-release syndrome occurred in about three-quarters of patients, cases were mostly grade 1 or 2, and it tapered off after the first dose. This ongoing phase I study will enroll 120 patients.
Dr. Anderson and his research team have studied the bispecific T-cell engager AMG 701 in combination with immunomodulatory drugs, and showed that this combination enhances both bispecific T-cell engager–mediated cytotoxicity and immunomodulation. In a mouse model, the combination of AMG 701 and lenalidomide or pomalidomide induced significantly greater myeloma cell regression than either agent alone, resulting in enhanced tumor regression and prevention of disease relapse.7
“As with targeted therapies, we are going to need to use combinations of immune therapies in myeloma,” Dr. Anderson commented. “When you combine bispecific T-cell engager and immunomodulatory drugs, you can use lower doses, which will likely result in a more favorable therapeutic index and more prolonged response.”
CAR T Cells
Moving through the pipeline in the BCMA-targeted CAR T-cell space are JNJ-4528, idecabtagene vicleucel, and orvacabtagene autoleucel.
JNJ-4528 is a bivalent BCMA CAR T-cell product, meaning it has antibody-binding domains at two different sites on BCMA, and it is linked to the costimulatory molecule 4-1BB. In a study of 29 patients with highly refractory disease reported at the 2019 ASH Annual Meeting & Exposition, the response rate was 100%; of 17 evaluable patients, 100% were minimal residual disease–negative at day 28; and in 27 of 29 patients, disease had not progressed at a median follow-up of 6 months.8
During the ASCO20 Virtual Scientific Program, data for these three leading candidates were updated: idecabtagene vicleucel (KarMMA trial), orvacabtagene autoleucel (EVOLVE trial), and JNJ-4528 (CARTITUDE trial).9-11 All studies were conducted in heavily pretreated patients with advanced disease, many of whom had triple-refractory disease. The objective response rates ranged from 73% with idecabtagene vicleucel to 92% with orvacabtagene autoleucel to 100% with JNJ-4528.9-11
“Minimal residual disease–negativity rates for these three CAR T-cell products are very, very high,” according to Dr. Anderson. “The overall response and extent of response are high, but median progression-free survival is on the order of 1 to 1.5 years,” he noted. “To date, we have not achieved very durable responses. We anxiously await more prolonged follow-up from ongoing studies, and hope to see more prolonged benefit.”
Cytokine-release syndrome is a common complication of BCMA CAR T-cell therapy, though most cases have been mild. “We are learning how to recognize and intervene early by interrupting cytokine-release syndrome,” explained Dr. Anderson.
The current CAR T-cell products are DNA-based. Dr. -Anderson’s group is studying BCMA mRNA-electroporated, CD8-positive CAR T cells, which are transiently active against myeloma cells. “The idea is to be able to use mRNA CAR T cells repeatedly, as we do with targeted therapies, and achieve deeper extent and duration of response,” he said.
DISCLOSURE: Dr. Anderson has served as a consultant to or received honoraria from Bristol Myers Squibb, Sanofi, Millennium, Gilead, Janssen, Tolero Pharmaceuticals, and Precision Biosciences and owns stock in C4 Therapeutics and OncoPep.
REFERENCES
3. Richardson PG, Vangsted AJ, Ramasamy K, et al: First-in-human phase I study of the novel CELMoD agent CC-92480 combined with dexamethasone in patients with relapsed/refractory multiple myeloma. ASCO20 Virtual Scientific Program. Abstract 8500.
4. Lonial S, Lee HC, Badros A, et al: Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol 21:207-221, 2020.
5. Lonial S, Lee HC, Badros A, et al: Pivotal DREAMM-2 study: Single-agent belantamab mafodotin (GSK2857916) in patients with relapsed/refractory multiple myeloma refractory to proteasome inhibitors, immunomodulatory agents, and refractory and/or intolerant to anti-CD38 monoclonal antibodies. ASCO20 Virtual Scientific Program. Abstract 8536.
9. Munshi NC, Anderson Jr LD, Jagannath S, et al: Idecabtagene vicleucel, a BCMA-targeted CAR T-cell therapy, in patients with relapsed and refractory multiple myeloma: Initial KarMMa results. ASCO20 Virtual Scientific Program. Abstract 8503.
10. Mailankody S, Jakubowiak AJ, Htut M, et al: Orvacabtagene autoleucel, a B-cell maturation antigen-directed CAR T cell therapy for patients with relapsed/refractory multiple myeloma: Update of the phase 1/2 EVOLVE study (NCT03430011). ASCO20 Virtual Scientific Program. Abstract 8504.
11. Berdeja JG, Madduri D, Usmani SZ, et al: Update of CARTITUDE-1: A phase Ib/II study of JNJ-4528, a B-cell maturation antigen–directed CAR T-cell therapy, in relapsed/refractory multiple myeloma. ASCO20 Virtual Scientific Program. Abstract 8505.

