
Scott Gettinger, MD

Naiyer A. Rizvi, MD
The phase I CheckMate 012 study examined the effects of nivolumab (Opdivo) monotherapy and nivolumab combined with platinum-based doublets as first-line treatments in patients with advanced non–small cell lung cancer (NSCLC). Encouraging efficacy and safety findings were reported in the Journal of Clinical Oncology by Scott Gettinger, MD, of Yale Cancer Center, and colleagues and by Naiyer A. Rizvi, MD, of Columbia University Medical Center, and colleagues.1,2
Nivolumab Monotherapy
As reported by Dr. Gettinger and colleagues,1 a total of 52 patients with stage IIIB to IV disease were treated with nivolumab at 3 mg/kg every 2 weeks until disease progression or unacceptable toxicity. Patents had a median age of 67 years; 50% were women; 94% had stage IV disease; 75% had nonsquamous histology; 15% had epidermal growth factor receptor (EGFR)-mutant tumors; 79% were former/current smokers; 40% had received prior radiotherapy; and 21% and 4% had received prior adjuvant and neoadjuvant platinum-based therapy, respectively. Treatment after disease progression was permitted.
Toxicity
Update on Nivolumab in Advanced Lung Cancer
- First-line nivolumab monotherapy was associated with durable responses, including complete responses, in patients with advanced NSCLC.
- Nivolumab in combination with platinum-based doublets was highly active as first-line treatment in patients with advanced NSCLC.
Treatment-related adverse events of any grade occurred in 71% of patients, with the most common being fatigue (29%), rash (19%), nausea (14%), diarrhea (12%), pruritus (12%), and arthralgia (10%). Grade 3 or 4 treatment-related adverse events occurred in 19%, with grade 3 rash being the most common (two patients, 4%). Study treatment was discontinued due to an adverse event in six patients (12%), including grade 4 increased alanine transaminase and grade 3 increased aspartate transaminase in one patient and cardiac failure, hyperglycemia, increased lipase, diarrhea,
and pneumonitis (all grade 3, one patient each).
Responses and Survival
Median follow-up was 14.3 months. Confirmed objective response occurred in 12 of 52 patients (23%), including ongoing complete response in 4 patients. Stable disease was observed in 27% of patients (disease control rate = 50%). Response was observed in 2 of 13 patients (15%) with squamous and 10 of 39 patients (26%) with nonsquamous histology. Response occurred by first tumor assessment at 11 weeks in 9 of 12 responders (75%). At the time of analysis, median duration of response had not been reached, with 8 responses ongoing at 5.3+ to 25.8+ months. Response was observed in 9 of 32 patients (28%) with any degree of tumor PD-L1 (programmed cell death ligand 1) expression and in 2 of 14 patients (14%) with no PD-L1 expression.
First-line nivolumab monotherapy demonstrated a tolerable safety profile and durable responses in first-line advanced NSCLC.— Scott Gettinger, MD, and colleagues
Tweet this quote
Median progression-free survival was 3.6 months (3.5 and 5.0 months for squamous and nonsquamous histology, respectively), and progression-free survival at 24 weeks was 41% (31% and 45% for squamous and nonsquamous histology, respectively). Median overall survival was 19.4 months (16.8 months and not reached for squamous and nonsquamous histology, respectively), with 1-year and 18-month rates of 73% (76% and 72%) and 57% (42% and 63%).
The investigators concluded: “First-line nivolumab monotherapy demonstrated a tolerable safety profile and durable responses in first-line advanced NSCLC.”
Nivolumab Added to Platinum-Based Doublets
Encouraging activity was observed, especially for the nivolumab 5 mg/kg plus paclitaxel-carboplatin group, with a 2-year overall survival rate of 62%.— Naiyer A. Rizvi, MD, and colleagues
Tweet this quote
As reported by Dr. Rizvi and colleagues,2 a total of 56 patients with stage IIIB or IV disease received nivolumab plus a platinum-based doublet every 3 weeks for 4 cycles followed by nivolumab alone until disease progression or unacceptable toxicity. Treatments consisted of nivolumab at 10 mg/kg plus gemcitabine/cisplatin (n = 12; for squamous histology) or pemetrexed (Alimta)/cisplatin (n = 15; nonsquamous histology) or nivolumab at 5 mg/kg (n = 14) or 10 mg/kg (n = 15) plus paclitaxel/carboplatin (all histologies). Patients had a median age of 64 years; 54% were women; 96% had stage IV disease; 86% were former/current smokers; 11% had EGFR-mutant tumors; 38% had prior radiotherapy; and 29% had received prior systemic therapy.
Toxicity
No dose-limiting toxicities were observed during the first 6 weeks of treatment. The most common treatment-related adverse events of any grade were fatigue (71%), nausea (46%), decreased appetite (36%), and alopecia (30%). Grade 3 or 4 treatment-related adverse events occurred in 45% of patients, with the most common being pneumonitis (7%), fatigue (5%), and acute renal failure (5%). Treatment-related adverse events led to discontinuation of all study therapy in 21% of patients.
Responses and Survival
Median follow-up was 19.0 months (Table 1). Objective response rates were 33% (disease control rate = 92%), 47% (disease control rate = 93%), 47% (73%), and 43% (86%) with nivolumab at 10 mg/kg plus gemcitabine/cisplatin, nivolumab at 10 mg/kg plus pemetrexed/cisplatin, nivolumab at 10 mg/kg plus paclitaxel/carboplatin, and nivolumab at 5 mg/kg plus paclitaxel/carboplatin, respectively. Responses were observed irrespective of tumor PD-L1 expression status. Median durations of response were 10.3, 5.8, 5.5, and 19.6 months.
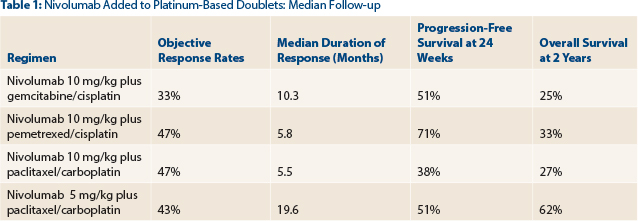
Progression-free survival at 24 weeks was 51%, 71%, 38%, and 51%, with nivolumab at 10 mg/kg plus gemcitabine/cisplatin, nivolumab at 10 mg/kg plus pemetrexed/cisplatin, nivolumab at 10 mg/kg plus paclitaxel/carboplatin, and nivolumab at 5 mg/kg plus paclitaxel/carboplatin, respectively. Two-year overall survival was 25%, 33%, 27%, and 62%, respectively.
The investigators concluded: “The safety profile of nivolumab plus [platinum-based doublet chemotherapy] was consistent with that expected for individual agents; however, treatment discontinuation related to [adverse events] was greater with the combination. Encouraging activity was observed, especially for the nivolumab 5 mg/kg plus paclitaxel-carboplatin group, with a 2-year overall survival rate of 62%.” ■
Disclosure: The study was supported by Bristol-Myers Squibb. For full disclosures of the study authors, visit www.jco.ascopubs.org.
References


