For advanced non–small cell lung cancer (NSCLC), first-line treatment with combined immune checkpoint blockade—in novel doses and schedules—was associated with deep and durable responses, encouraging progression-free survival, and much better tolerability than has been previously observed with nivolumab (Opdivo) plus ipilimumab (Yervoy).
The results are from the CheckMate 012 trial and were presented at the 16th World Conference on Lung Cancer in Denver by Naiyer Rizvi, MD, Director of Thoracic Oncology and Immunotherapeutics in Medical Oncology at NewYork–Presbyterian/Columbia University Medical Center.1 The annual meeting is hosted by the International Association for the Study of Lung Cancer.
CheckMate 012 Details
CheckMate 012 compared several dosing schedules for combining the programmed cell death protein 1 (PD-1) antibody nivolumab and the CTLA4 inhibitor ipilimumab for front-line use in treating advanced NSCLC.
“We think that nivolumab at 3 mg/kg every 2 weeks plus ipilimumab at 1 mg/kg every 6 or 12 weeks are the best schedules and the ones to move forward with,” Dr. Rizvi told The ASCO Post.
Previous phase III studies have shown an overall survival benefit for nivolumab, as compared to docetaxel, in previously treated patients with advanced NSCLC.
“We decided to explore the safety and efficacy of nivolumab in the first-line setting, not just as monotherapy but combined with other therapies, really pushing the envelope about where we should go next with immune checkpoint blockade,” Dr. Rizvi said.
CheckMate 012 studied nivolumab alone and in various combinations that involved platinum-based chemotherapy, erlotinib, bevacizumab (Avastin), and ipilimumab. The combination with ipilimumab mirrored the dose and schedules used in metastatic melanoma (nivolumab at 1 mg/kg plus ipilimumab at 3 mg/kg every 3 weeks or nivolumab at 3 mg/kg plus ipilimumab at 1 mg/kg every 3 weeks). Response rates with these combinations were 13% and 20%, respectively.
Safety and Tolerability
Grade 3/4 treatment-related adverse event rates were 58% and 44%, and 37% of patients discontinued this combination due to toxicity.
“The toxicity was more than that seen with single-agent nivolumab and more than we have observed with the combination in melanoma. It was clear that lung cancer behaves differently, and different doses and schedules needed to be explored,” he said.
With safety and tolerability as the primary endpoint, Dr. Rizvi and colleagues therefore evaluated several new dosing schedules for this combination:
- Nivolumab at 1 mg/kg every 3 weeks × 4 plus ipilimumab at 1 mg/kg every 3 weeks × 4 plus nivolumab at 3 mg/kg every 2 weeks (n = 31)
- Nivolumab at 1 mg/kg every 2 weeks plus ipilimumab at 1 mg/kg every 6 weeks (n = 40)
- Nivolumab at 3 mg/kg every 2 weeks plus ipilimumab at 1 mg/kg every 12 weeks (n = 38)
- Nivolumab at 3 mg/kg every 2 weeks plus ipilimumab at 1 mg/kg every 6 weeks (n = 39).
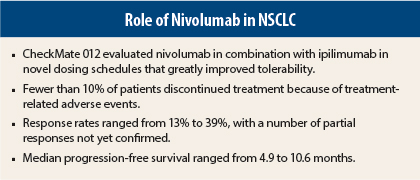
“We found that the combination was very well tolerated in all the cohorts,” Dr. Rizvi said. “Few patients (3%–10%) discontinued treatment due to adverse events. This compares favorably to what we see with first-line monotherapy with nivolumab (10%).”
Grade 3/4 treatment-related adverse events occurred in 28% to 35% of patients and were “all quite manageable.” There were no treatment-related deaths.
“The toxicity profile is very different from our initial experience with this combination,” Dr. Rizvi said.
High Response Rates
“First-line therapy with nivolumab plus ipilimumab demonstrated a high level of clinical activity characterized by deep and durable responses in advanced lung cancer,” Dr. Rizvi reported.
Objective responses were confirmed in 13% to 39%, with additional unconfirmed partial responses observed. The highest response rate, 39%, occurred in the cohort receiving nivolumab at 3 mg/kg every 2 weeks plus ipilimumab at 1 mg/kg every 12 weeks.
Efficacy by Tumor PD-L1 Expression
Clinical activity was observed and those with tumors expressing programmed cell death ligand type 1 (PD-L1) had better responses than those whose tumors did not express PD-L1. The majority of all responders were still responding at data cutoff.
In patients with ≥ 1% PD-L1 expression, response rates ranged from 8% (nivolumab at 1 mg/kg every 3 weeks × 4 plus ipilimumab at 1 mg/kg every 3 weeks × 4) to 48% (nivolumab at 3 mg/kg every 2 weeks plus ipilimumab at 1 mg/kg every 12 weeks, and nivolumab at 3 mg/kg every 2 weeks plus ipilimumab at 1 mg/kg every 6 weeks).
The response rate was higher in the PD-L1 positive vs PD-L1 negative cohorts overall but the majority of patients had PD-L1 expression greater than or equal to 1% (about 70%).
Median duration of response was not reached in any arm, regardless of PD-L1 expression. Median progression-free survival was not reached among PD-L1–positive patients receiving nivolumab at 3 mg/kg every 2 weeks plus ipilimumab at 1 mg/kg every 6 weeks and was 34.8 weeks for the cohort receiving nivolumab at 3 mg/kg every 2 weeks plus ipilimumab at 1 mg/kg every 12 weeks, which appeared longer than for PD-L1–negative patients in these cohorts.
A phase III trial, CheckMate 227, is currently comparing nivolumab or nivolumab plus ipilimumab vs platinum-based doublet chemotherapy in chemotherapy-naive stage IV or recurrent NSCLC. ■
Disclosure: Dr. Rizvi received honoraria from Bristol-Myers Squibb, Roche, AstraZeneca, Merck, and Novartis.
Reference
1. Rizvi NA, Gettinger SN, Goldman JW, et al: Safety and efficacy of first-line nivolumab and ipilimumab in non-small cell lung cancer. 16th World Conference on Lung Cancer. Abstract ORAL02.05. Presented September 7, 2015.