The standard of care for malignant pleural mesothelioma may be poised for change, judging by results from a study by the French Cooperative Thoracic Intergroup. The addition of bevacizumab (Avastin) in the first-line setting to the current standard of care, pemetrexed (Alimta)/cisplatin, improved overall survival by almost 3 months in the phase III MAPS trial,1 according to a presentation at the 16th World Conference on Lung Cancer. The findings were reported by Arnaud Scherpereel, MD, Head, Pulmonary and Thoracic Oncology Department and Professor, University Hospital, Lille, France.
“Adding bevacizumab to pemetrexed/cisplatin doublet significantly increased both progression-free survival (by 2 months) and overall survival (by 2.75 months), with only a slight, manageable increase of toxicity,” Dr. Scherpereel said.
Mesothelioma is a very aggressive tumor with no validated curative treatment. Maximum overall survival hovers around 13 months, and “no improvement has been achieved in this disease in more than a decade,” he noted. According to Dr. Scherpereel, the scenario has changed with the results of MAPS, which could herald “a new treatment paradigm” for patients with mesothelioma who are eligible for bevacizumab and are not candidates for potentially curative surgery.
Study Details
MAPS is a phase II/III open-label, 73-center study conducted between 2008 and 2014. It enrolled 448 patients with mesothelioma who were not amenable to curative treatment, randomizing them to standard chemotherapy with pemetrexed/cisplatin or the same regimen plus bevacizumab (15 mg/kg). After six cycles, patients on the experimental arm continued to receive bevacizumab alone until disease progression.
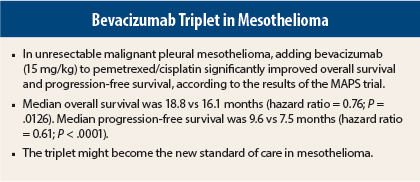
Efficacy, Safety of the Triplet
After a median follow-up of 39.4 months, the primary endpoint, median overall survival, was significantly higher in the experimental arm receiving triplet therapy (18.8 vs 16.1 months; hazard ratio [HR] = 0.76; P = .015). Median progression-free survival was also significantly longer with the triplet (9.6 vs 7.5 months; HR = 0.61; P < .0001). The median overall survival of the control arm was longer than has been observed in historical series or previous trials, perhaps because study subjects were unusually fit, Dr. Scherpereel said.
Grade 3/4 toxicities were observed in 71% of the bevacizumab arm and 62% of the control arm. Hematologic toxicities were not increased with bevacizumab, but nonhematologic adverse events were greater with triplet therapy, including a higher incidence of creatinine elevations (39% vs 28%), hypertension (56% vs 1%), arterial and venous thromboembolic events (7% vs 1%), and low-grade hemorrhage (41% vs 7%).
There was no significant difference between the arms in terms of percent of drug delivered or proportion of patients receiving second-line treatment. Therefore, these factors do not explain bevacizumab’s benefits, he said. ■
Disclosure: Dr. Scherpereel reported that Roche provided bevacizumab for the trial and his research lab received a grant in 2012–2015.
Reference
1. Scherpereel A, Mazières J, Margery J, et al: 16th World Conference on Lung Cancer. Abstract 2142. Presented September 7, 2015.