The antibody-drug conjugate brentuximab vedotin (Adcetris) was granted accelerated approval on August 19 for the treatment of relapsed or refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Brentuximab vedotin is the first new drug to be approved in Hodgkin lymphoma in more than 30 years.
In Hodgkin lymphoma, the drug is indicated for use in patients with progressive disease after autologous stem cell transplantation and in those ineligible for transplantation after at least two multiagent chemotherapy regimens have failed. In anaplastic large cell lymphoma, it is indicated for use in patients after failure of at least one prior chemotherapy treatment.
“Early clinical data suggest that patients who received [brentuximab vedotin] for Hodgkin lymphoma and systemic anaplastic lymphoma experienced a significant response to the therapy,” said Richard Pazdur, MD, Director of the Office of Oncology Drug Products in the FDA’s Center for Drug Evaluation and Research.
Mechanism of Action
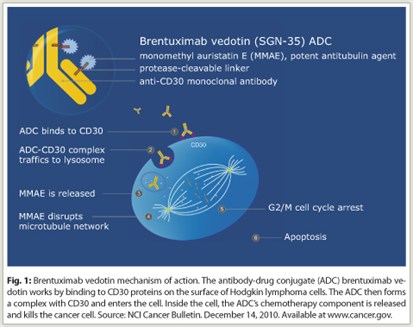 CD30, a defining marker of Hodgkin lymphoma, is expressed on the surface of Hodgkin Reed-Sternberg cells as well as cells in anaplastic large cell lymphomas, embryonal carcinomas, and select subtypes of B-cell-derived non-Hodgkin lymphomas and mature T-cell lymphomas. The restriction of normal expression of CD30 to this relatively small population of activated B cells and T cells and a small portion of eosinophils makes the molecule an attractive therapeutic target. Anti-CD30 antibodies alone have thus far shown little clinical activity in Hodgkin lymphoma.
CD30, a defining marker of Hodgkin lymphoma, is expressed on the surface of Hodgkin Reed-Sternberg cells as well as cells in anaplastic large cell lymphomas, embryonal carcinomas, and select subtypes of B-cell-derived non-Hodgkin lymphomas and mature T-cell lymphomas. The restriction of normal expression of CD30 to this relatively small population of activated B cells and T cells and a small portion of eosinophils makes the molecule an attractive therapeutic target. Anti-CD30 antibodies alone have thus far shown little clinical activity in Hodgkin lymphoma.
Brentuximab vedotin is formed from the anti-CD30 monoclonal antibody brentuximab and the antitubulin agent monomethyl auristatin E (vedotin), joined by an enzyme-cleavable dipeptide linker. The conjugate is designed to be stable in the circulation. After binding to CD30 via brentuximab, the conjugate is rapidly internalized and transported to lysosomes, wherein the linker is selectively cleaved. Vedotin is released into the cell and binds to tubulin, resulting in breakdown of microtubules, G2/M (gap 2 phase and mitosis) cell-cycle arrest, and apoptosis (Fig. 1).
Two Pivotal Trials
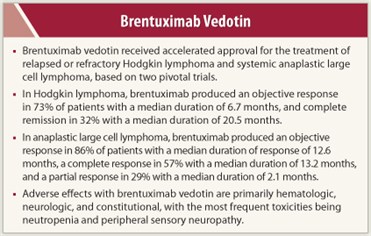 Approval of brentuximab vedotin in both settings was based on findings in two pivotal, open-label, single-group clinical trials in which overall response rate was assessed by an independent review facility as the primary endpoint. Brentuximab vedotin was administered intravenously at a dose of 1.8 mg/kg over 30 minutes once every 3 weeks. The study in Hodgkin lymphoma included 102 patients (median age, 31 years; range, 15 to 77 years; 53% female, 87% white) who had received a median of five prior therapies including autologous stem cell transplantation. Objective response occurred in 73% of patients, with a median duration of response of 6.7 months. Complete remission occurred in 32%, with a median response duration of 20.5 months and responses that were ongoing at the time of the analysis (range, 1.4 to 21.9+ months), and partial remission occurred in 40%, with a median duration of 3.5 months (range, 1.3 to 18.7 months).
Approval of brentuximab vedotin in both settings was based on findings in two pivotal, open-label, single-group clinical trials in which overall response rate was assessed by an independent review facility as the primary endpoint. Brentuximab vedotin was administered intravenously at a dose of 1.8 mg/kg over 30 minutes once every 3 weeks. The study in Hodgkin lymphoma included 102 patients (median age, 31 years; range, 15 to 77 years; 53% female, 87% white) who had received a median of five prior therapies including autologous stem cell transplantation. Objective response occurred in 73% of patients, with a median duration of response of 6.7 months. Complete remission occurred in 32%, with a median response duration of 20.5 months and responses that were ongoing at the time of the analysis (range, 1.4 to 21.9+ months), and partial remission occurred in 40%, with a median duration of 3.5 months (range, 1.3 to 18.7 months).
The study in systemic anaplastic large cell lymphoma included 58 patients (median age, 52 years; range, 14 to 76 years; 57% male, 83% white) who had relapsed after (50%) or were refractory on (50%) their most recent prior therapy. Patients had received a median of two prior therapies, with 26% having received prior autologous stem cell transplantation. Approximately 72% of patients were anaplastic lymphoma kinase (ALK)-negative. Objective response occurred in 86% of patients, with a median duration of response of 12.6 months. Some responses, including complete and partial responses, were ongoing at the time of analysis (range, 0.1 to 15.9+ months). Complete response occurred in 57%, with a median duration of response of 13.2 months (range, 0.7 to 15.9+ months), and partial response occurred in 29%, with a median duration of 2.1 months (range, 0.1 to 15.8+ months).
Toxicities
Adverse effects with brentuximab vedotin are primarily hematologic, neurologic, and constitutional. In Hodgkin lymphoma, the most common adverse events (> 20%) were neutropenia (54%), peripheral sensory neuropathy (52%), fatigue (49%), upper respiratory tract infection (47%), nausea (42%), diarrhea (36%), anemia (33%), pyrexia (29%), thrombocytopenia (28%), rash (27%), abdominal pain (25%), cough (25%), and vomiting (22%). The most common grade 3/4 events were neutropenia (15%/6%), anemia (8%/2%), thrombocytopenia (7%/2%), peripheral sensory neuropathy (8% grade 3), and peripheral motor neuropathy (4% grade 3).
In systemic anaplastic large cell lymphoma, the most frequent adverse events were neutropenia (55%), peripheral sensory neuropathy (53%), anemia (52%), fatigue (41%), nausea (38%), pyrexia (38%), rash (31%), diarrhea (29%), and pain (28%). The most common grade 3/4 events were neutropenia (12%/9%), thrombocytopenia (5%/5%), peripheral sensory neuropathy (10% grade 3), pain (5% grade 4), fatigue (2%/2%), and pain in extremity (2%/2%).
Ongoing Trials
Several studies of brentuximab vedotin are ongoing. The AETHERA trial (ADC Empowered Trial for Hodgkin to Evaluate PRogression after ASCT) is a phase III randomized, double-blind trial comparing brentuximab vedotin plus best supportive care vs placebo plus best supportive care in patients with Hodgkin lymphoma at high risk of relapse after autologous stem cell transplantation. A phase II trial is evaluating the potential for retreatment with brentuximab vedotin in patients who have relapsed after discontinuing previous brentuximab vedotin therapy.
A phase I, two-arm, open-label, dose-escalation study is investigating the combination of brentuximab vedotin with multiagent chemotherapy in front-line treatment of Hodgkin lymphoma. The treatment arms consist of brentuximab vedotin in combination with ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) or with AVD (doxorubicin, vinblastine, dacarbazine). Another phase I trial is evaluating brentuximab vedotin given sequentially and concurrently with multiagent chemotherapy as front-line treatment in patients with systemic anaplastic large cell lymphoma. Brentuximab vedotin is being given sequentially with cyclophosphamide, prednisone, doxorubicin, and vincristine and in concurrent and sequential combination with cyclophosphamide, doxorubicin, and prednisone. ■
SIDEBAR: Important Benefit for Small Population Is a Major Milestone in Lymphoma
[What Will It Cost?]

 The approval of brentuximab vedotin (Adcetris) is a major milestone for the treatment of patients with relapsed Hodgkin lymphoma and anaplastic large cell lymphoma. It represents an excellent example of personalized cancer therapy. Patients are preselected based on a predictive biomarker that is...
The approval of brentuximab vedotin (Adcetris) is a major milestone for the treatment of patients with relapsed Hodgkin lymphoma and anaplastic large cell lymphoma. It represents an excellent example of personalized cancer therapy. Patients are preselected based on a predictive biomarker that is...