“I think the biggest innovations of the 21st century will be at the intersection of biology and technology. A new era is beginning!” —Steve Jobs
Relapsed and refractory multiple myeloma remains a treatment challenge. Promising responses have been demonstrated with bispecific antibodies, with a potential for improved outcomes in this setting. Here is a roundup of recently published or presented studies of these agents, illustrating some of these promising data.
Teclistamab
MajesTEC-1 Study: A phase I/II study of teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in relapsed or refractory multiple myeloma (ClinicalTrials.gov identifier NCT04557098).1
Background: Teclistamab is a bispecific IgG4 antibody that binds B-cell maturation antigen (BCMA) and CD3 to redirect T cells to multiple myeloma cells. In the phase I study, 165 patients who had received a median of six prior lines of therapy received teclistamab intravenously (0.3–19.2 μg/kg once every 2 weeks or 19.2–720 μg/kg once per week) or subcutaneously (80–3,000 μg/kg once per week), with step-up dosing for doses ≥ 38.4 μg/kg. The recommended phase II dose was identified as teclistamab given subcutaneously at 1,500 μg/kg once per week after 60 μg/kg and 300 μg/kg step-up doses.2
GUEST EDITORS

Syed Ali Abutalib, MD

Saad Z. Usmani, MD, MBA, FACP
Dr. Abutalib is Assistant Director, Hematology and Hematopoietic Cell Transplantation; Director, Hematopoietic Cell Transplant Apheresis Service, Cancer Treatment Centers of America, Zion, Illinois; Founder and Co–Editor-in-Chief, Advances in Cell & Gene Therapy. Dr. Usmani is Professor of Medicine; Chief, Myeloma Service, Memorial Sloan -Kettering Cancer Center, New York.
The phase II data among 40 patients receiving the recommended phase II dose showed at least a partial response in 26 patients (overall response rate [ORR] = 65%; 95% confidence interval [CI] = 48%–79%), with at least a very good partial response in 23 (ORR = 58%; 95% CI = 43%–75%), at least a complete response in 16 (ORR = 40%; 95% CI = 25%–57%), and at least a stringent complete response in 7 (ORR = 18%). The median time to a first confirmed response was 1.0 month. The median duration of response was not reached (95% CI = 7.2 months to not reached), with 22 of 26 responders (85%) remaining alive and continuing treatment after a median follow-up of 7.1 months (interquartile range [IQR] = 5.1–9.1 months).2
Methods: Investigators reported additional data from the phase II portion of MajesTEC-1 and updated results for phase I patients treated at the phase II dose (n = 40). The primary endpoint in phase II (n = 125) is overall response rate to teclistamab at the phase II dosing level. None of the patients had prior anti-BCMA–based therapy.
Results:
Efficacy: At a median follow-up of 7.8 months (range = 1.2–15.2 months), response rates in the 165 phase I/II patients treated were consistent with previously presented data. At a median follow-up of 7.8 months (range = 0.5–18 months), an overall response rate of 62.0% (95% CI = 53.7%–69.8%) represents a substantial benefit for patients with triple-class–exposed disease. The median time to first response was 1.2 months (range = 0.2–5.5 months). Measurable residual disease (MRD) negativity rate was 24.7% (37/150; 95% CI = 18.0%–32.4%) at a threshold of 10–5 and 16.7% (25/150; 95% CI = 11.1%–23.6%) at a threshold of 10–6. In patients who achieved a complete response, the MRD negativity rate was 41.9%. The median duration of response and median progression-free survival have not been reached. The 6-month progression-free survival rate is 64.4% (95% CI = 56.0%–71.7%), and the 9-month progression-free survival rate is 58.5% (95% CI = 48.8%–67.0%).
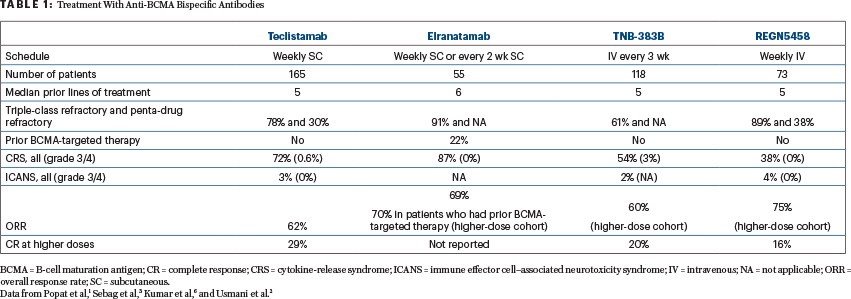
Adverse Events: The most common hematologic adverse event was pancytopenia. The most common nonhematologic adverse events in all 165 patients were cytokine-release syndrome (71%; grade 1/2 = 99%; 1 patient had a transient grade 3 event; median time to onset = 2 days [range = 1–6 days]; median duration = 2 days [range = 1–9 days]), injection-site erythema (25.5%; all grade 1/2), and fatigue (25%; grade 3/4 = 2%). A total of 21 patients (12.7%) developed neurotoxicity (all grade 1/2; all resolved), with headache being the most common (n = 14).
Clinical Implications: Data from MajesTEC-1 continue to show that weekly teclistamab induces deep and durable responses in heavily pretreated patients with relapsed or refractory multiple myeloma, with low rates of grade 3 or 4 cytokine-release syndrome (0.6%) and minimal neurotoxicity. The subcutaneous route of administration allows higher doses than the intravenous method without increasing—and possibly mitigating—the incidence of cytokine-release syndrome. Most recently, a comprehensive report of these and additional findings of this trial were published in TheNew England Journal of Medicine.3
Teclistamab is also being evaluated in several combination studies (NCT04586426, NCT04108195, NCT04722146, NCT05083169). In 2020, the European Commission and the U.S. Food and Drug Administration (FDA) both granted teclistamab Orphan Drug designation for the treatment of multiple myeloma. In January 2021 and June 2021, teclistamab received a Priority Medicines (PRIME) designation from the European Medicines Agency (EMA) and Breakthrough Therapy designation from the FDA.
Elranatamab
MagnetisMM-1 Study: Elranatamab in patients with relapsed or refractory multiple myeloma. Results from a phase I study (NCT03269136).4
Background: Elranatamab is a humanized bispecific molecule that targets BCMA expressed on multiple myeloma and engages CD3 on T cells, for patients with relapsed or refractory multiple myeloma.
Methods: The investigators reported results from the subcutaneous cohorts: dose escalation (part 1), monotherapy with priming (part 1.1; total of 48 patients in part 1 and 1.1), lenalidomide combination (part 1C; n = 4), pomalidomide combination (part 1D; n = 4), and monotherapy expansion with priming (part 2A).
In part 1, patients received elranatamab at 80, 130, 215, 360, 600, and 1,000 μg/kg weekly. For monotherapy at the recommended phase II dose (parts 1.1 and 2A), a single priming dose (600 μg/kg or equivalent fixed dose of 44 mg) was followed 1 week later by the full dose (1,000 μg/kg or equivalent fixed dose of 76 mg) weekly or every 2 weeks thereafter. For lenalidomide and pomalidomide combination therapy, a single priming dose (32 mg) was followed 1 week later by the full dose (44 mg) weekly thereafter in combination with either lenalidomide or pomalidomide (4 mg) on days 1 to 21 of a 28-day cycle.
Patients had received a median of six prior regimens, 98% had triple-class relapsed or refractory disease, 45% had prior high-dose chemotherapy with stem cell transplantation, and 22% had prior BCMA-targeted therapy. The median duration of follow-up was 7.5, 2.3, and 1.9 months for the dose-escalation, priming, and combination cohorts, respectively.
Results: The most common treatment-emergent adverse events were cytokine-release syndrome (83%; none higher than grade 2), cytopenias, and injection-site reaction. At the recommended phase II dose of 1,000 μg/kg, the median duration of cytokine-release syndrome decreased by 50%, from 4 days to 2 days, with priming. Of 58 patients, 2 had dose-limiting toxicity, including grade 4 thrombocytopenia (part 1.1) and grade 4 neutropenia (pomalidomide). See Table 1 for additional efficacy and toxicity data.
Clinical Implications: Elranatamab as a single agent achieved a complete response rate (including stringent remissions) of 30%. Of note, elranatamab induced deep and durable clinical responses in patients with relapsed or refractory multiple myeloma, with and without prior BCMA-targeted therapy. These results, along with emerging combination data, support the continued development of -elranatamab.
REGN5458
First-in-Human Study: Early, deep, and durable responses, and low rates of cytokine-release syndrome, with REGN5458 given by intravenous infusion, in a phase I/II first-in-human study in relapsed or refractory multiple myeloma (NCT03761108).5
Background: Investigators have previously presented preliminary data from an ongoing phase I/II trial demonstrating that REGN5458 monotherapy had an acceptable safety and tolerability profile with early, deep, and durable responses in heavily pretreated patients, with at least triple-refractory relapsed or refractory multiple myeloma.6 Here we describe updated safety, overall response, and response durability in patients treated in the phase I portion of this study.
Methods: Patients with progressive relapsed or refractory multiple myeloma, who were triple-refractory or intolerant to prior lines of systemic therapy including a proteasome inhibitor, immunomodulatory agent, and anti-CD38 antibody, were treated with REGN5458 monotherapy following a modified 3+3 dose-escalation design (4+3). Treatment consisted of 16 weekly infusions of REGN5458, followed by every-2-week dosing, until disease progression. The median duration of follow-up was 2.4 months (range = 0.1–20.8 months; Table 1).
Results:
Treatment-Emergent Adverse Events were reported in 97.1%, with at least three such events in 76.5% of patients. The most frequent adverse events were fatigue and cytokine-release syndrome (Table 1). No patient had grade ≥ 3 cytokine-release syndrome or discontinued treatment because of cytokine-release syndrome. There were no grade ≥ 3 neurotoxicity events. Nausea was reported in 32.4%. The severity of nausea was grade 1 in 23.5% of patients and grade 2 in 8.8% of patients.
Treatment-Related Adverse Events were reported in 82.4%, the most frequent being hematologic (neutropenia in 16.2%, with grade ≥ 3 severity in 3.2%). The most frequent nonhematologic adverse events were cytokine-release syndrome (38.2%) and fatigue (20.6%). The safety profile was consistent across all dose levels, and there was no correlation between cytokine-release syndrome and the full dose of REGN5458.
Efficacy: Responses were observed at all dose levels. Among patients treated at the 96-mg and 200-mg doses, the response rate was 73.3% (11 of 15 patients). The Kaplan-Meier estimated median duration of response was not reached, and the probability of response duration of at least 8 months was 92.1% (95% CI = 72.1%–98.0%), with responses ongoing at up to 19 months per the latest data cutoff. Disease response was not impacted by the level of BCMA expression in the core biopsy, as assessed by immunohistochemistry.
Clinical Implications: In this updated analysis of the first-in-human study, REGN5458 continues to show an acceptable safety and tolerability profile, with grade 2 cytokine-release syndrome in 4.4% of patients and no grade ≥ 3 cytokine-release syndrome or neurotoxicity events. No new safety signals were observed during the additional follow-up period. Results seen with REGN5458 as well as data from studies of two other anti-BCMA bispecific antibodies, elranatamab (NCT03269136)4 and TNB-383B (NCT03933735),7 are summarized in Table 1.
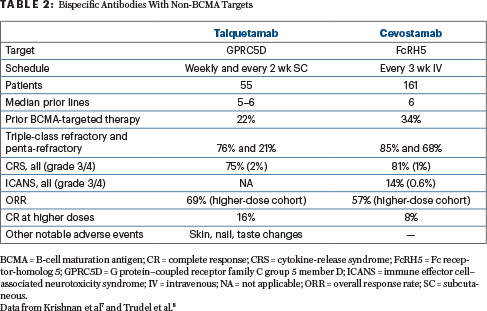
Talquetamab
MonumenTAL-1 Study: First-in-human study of talquetamab monotherapy in patients with relapsed or refractory multiple myeloma: Updated phase I results (NCT03399799).8
Background: Talquetamab is a first-in-class bispecific IgG4 antibody that redirects T cells to kill myeloma cells by binding to both G protein–coupled receptor family C group 5 member D (GPRC5D) and CD3 receptors. GPRC5D has high expression on malignant plasma cells, with limited expression on normal human tissue; unlike BCMA antigen, there is no indication that GPRC5D sheds into the periphery.
Methods: Investigators updated and provided new results of talquetamab at the recommended phase II doses from a phase I trial in relapsed or refractory multiple myeloma. A total of 30 patients received the 405-μg/kg weekly dosing schedule—100% of these patients were triple-class exposed, and 80% were penta-drug exposed; 77% were triple-class refractory, 20% were penta-drug refractory; and 30% had received prior BCMA-directed therapy. A total of23 patients received the 800-μg/kg biweekly dosing schedule—96% of these patients were triple-class exposed, and 70% were penta-drug exposed; 65% were triple-class refractory, 22% penta-drug refractory; and 17% had received prior BCMA-directed therapy.
Results:
Adverse Events: There were no treatment discontinuations due to adverse events at either of the recommended phase II doses. The most common adverse events at the 405-μg/kg weekly dose were cytokine-release syndrome (73%; 1 patient had grade 3 cytokine-release syndrome), neutropenia (67%; grade 3/4 in 60%), and dysgeusia (60%; grade 2 in 29%); skin-related adverse events occurred in 77% of patients (all grade 1/2; nail disorders in 30%), and infections occurred in 37% of patients (1 patient had grade 3 COVID-19 pneumonia). The most common adverse events at the 800-μg/kg biweekly dose were cytokine-release syndrome (78%; all grade 1/2), dry mouth (44%; all grade 1/2), and neutropenia (44%; grade 3/4 in 35%). Skin-related adverse events occurred in 65% of patients (grade 3 in 13%; nail disorders in 17%), and infections occurred in 13% (1 patient had grade 3 pneumococcal sepsis).
Efficacy: In 30 response-evaluable patients treated with the 405-μg/kg weekly dose, the overall response rate was 70% (very good partial response or better = 57%). In 17 response-evaluable patients treated with the 800-μg/kg biweekly dose, the overall response rate was 71% (very good partial response or better = 53%). Responses were durable and deepened over time in both cohorts. The median duration of response was not reached at either of the recommended phase II doses; the 6-month duration of response rate for patients who received the 405-μg/kg weekly dose was 67% (95% CI = 41%–84%). Additional results of talquetamab and of cevostamab, a novel bispecific against FcRH5 (Fc receptor-homolog 5) antigen (NCT03275103),9 are summarized in Table 2.
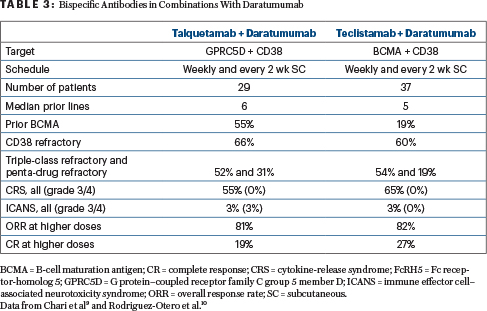
Clinical Implications: Subcutaneous talquetamab is well tolerated and highly effective at both recommended phase II doses. Preliminary data from the 800-μg/kg biweekly cohorts indicate that less frequent, higher doses of subcutaneous talquetamab do not have a negative impact on the previously described safety profile. Further investigation of talquetamab as monotherapy (phase II; NCT04634552) and in combination with daratumumab in patients with relapsed or refractory multiple myeloma is underway. Table 3 shows results with talquetamab (anti-GPRC5D) and teclistamab (anti-BCMA), in combination with daratumumab (anti-CD38), as demonstrated in MajesTEC-3 (NCT05083169) and TRIMM-2 (NCT04108195), respectively.10,11 A study of the combination of talquetamab and teclistamab in patients with relapsed or refractory multiple myeloma is open as well (NCT04586426).
DISCLOSURE: Dr. Abutalib has served on the advisory board for AstraZeneca. Dr. Usmani has received research funding from Amgen, Array Biopharma, BMS, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDx, Takeda; consulting fees from Amgen, BMS, Celgene, Edo Pharma, GSK, Janssen, Oncopeptides, Sanofi, Seattle Genetics, Secura Bio, SkylineDx, Takeda, TeneoBio; and speaker fees from Amgen, BMS, Janssen, and Sanofi.
REFERENCES
1. Popat R, Usmani S, Garfall A, et al: Updated results from the phase 1/2 MajesTEC-1 study of teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in relapsed/refractory multiple myeloma. Hemasphere 6(suppl):Abstract P06, 2022.
2. Usmani SZ, Garfall AL, van de Donk NWCJ, et al: Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): A multicentre, open-label, single-arm, phase 1 study. Lancet 398:665-674, 2021.
3. Moreau P, Garfall A, van de Donk N, et al: Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med 387:495-505, 2022.
4. Sebag M, Raje N, Bahlis N, et al: Elranatamab (PF-06863135), a B-cell maturation antigen targeted CD3-engaging bispecific molecule, for patients with relapsed or refractory multiple myeloma: Results from MagnetisMM-1. Blood 138(suppl 1):895, 2022.
5. Zonder JA, Richter J, Bumma N, et al: Early, deep, and durable responses, and low rates of cytokine-release syndrome with REGN5458, a BCMA × CD3 bispecific monoclonal antibody, in a phase 1/2 first-in-human study in patients with relapsed/refractory multiple myeloma. 2022 European Hematology Association Congress. Abstract S189.
6. Madduri D, Rosko A, Brayer J, et al: REGN5458, a BCMA × CD3 bispecific monoclonal antibody, induces deep and durable responses in patients with relapsed/refractory multiple myeloma. 2020 American Society of Hematology Annual Meeting & Exposition. Abstract 291.
7. Kumar S, D’Souza A, Shah N, et al: A phase 1 first-in-human study of Tnb-383B, a BCMA × CD3 bispecific T-cell redirecting antibody, in patients with relapsed/refractory multiple myeloma. Blood 138(suppl 1):900, 2021.
8. Krishnan AY, Minnema MC, Berdeja JG, et al: Updated phase 1 results from MonumenTAL-1: First-in-human study of talquetamab, a g protein-coupled receptor family C group 5 member D × CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma. Blood 138(suppl 1):653, 2021.
9. Trudel S, Cohen A, Krishnan A, et al: Cevostamab monotherapy continues to show clinically meaningful activity and manageable safety in patients with heavily pre-treated relapsed/refractory multiple myeloma: Updated results from an ongoing phase I study. Blood 138(suppl 1):157, 2021.
10. Chari A, Hari P, Bahlis N, et al: Phase 1b results for subcutaneous talquetamab plus daratumumab in patients with relapsed/refractory multiple myeloma. Blood 138(suppl 1):161, 2021.
11. Rodriguez-Otero P, Dholaria B, Askari E, et al: Subcutaneous teclistamab in combination with daratumumab for the treatment of patients with relapsed/refractory multiple myeloma: Results from a phase 1b multicohort study. Blood 138(suppl 1):1647, 2021.
Dr. Abutalib is Co-Director, Hematology and BMT/Cellular Therapy Programs; Director, Clinical Apheresis Programs; Cancer Treatment Centers of America, Zion, Illinois; Associate Professor, Rosalind Franklin University of Medicine and Science; and Founder and Co-Editor of Advances in Cell and Gene Therapy. Dr. Usmani is Professor of Medicine and Chief of the Myeloma Service, Memorial Sloan Kettering Cancer Center, New York, New York.

