As first-line treatment of advanced esophageal squamous cell carcinoma, nivolumab-containing regimens improved overall survival over standard-of-care chemotherapy, according to the first results of the global phase III CheckMate 648 trial presented at the 2021 ASCO Annual Meeting by Ian Chau, MD, Consultant Medical Oncologist at the Royal Marsden Hospital in the United Kingdom.1
Compared with patients receiving chemotherapy alone, the mortality risk was reduced in the primary PD-L1–positive population by 46% with nivolumab plus chemotherapy and by 36% with nivolumab plus ipilimumab. Relative risk was reduced by 26% and 22%, respectively, in all randomly assigned patients.

“Based on CheckMate 648, the largest randomized controlled trial in [esophageal squamous cell carcinoma], both [nivolumab-based] regimens represent potential new first-line standards of care.”— Ian Chau, MD
Tweet this quote
“Nivolumab is the first PD-1 inhibitor to demonstrate superior overall survival and durable responses in combination with either chemotherapy or ipilimumab, vs chemotherapy alone, in previously untreated patients with advanced esophageal squamous cell carcinoma,” Dr. Chau said. “Based on CheckMate 648, the largest randomized controlled trial in this disease setting, both regimens represent potential new first-line standards of care.”
‘Moving the Dial’
David Cunningham, MD, Consultant Medical Oncologist, Head of the Gastrointestinal and Lymphoma Unit, and Director of Clinical Research at The Royal Marsden in London and Surrey, United Kingdom, called the study “fantastic” and said the findings mean “we are moving the dial on this disease.”
ASCO Chief Medical Officer and Executive Vice President Julie R. Gralow, MD, FACP, FASCO,told journalists in a press briefing: “The dual immunotherapy combination is the first chemotherapy-free first-line treatment to show benefit in these patients…. The addition of the PD-1 immune checkpoint inhibitor to chemotherapy or ipilimumab should be considered superior first-line treatments for esophageal squamous cell carcinoma.”
About CheckMate 648
CheckMate 648 attempted to improve upon the poor prognosis of current first-line treatments, which yield median overall survival times of about 10 months. The study randomly assigned 970 patients irrespective of PD-L1 expression to one of three treatment arms: nivolumab at 240 mg every 2 weeks plus chemotherapy with fluorouracil and cisplatin every 4 weeks; (2) nivolumab at 3 mg/kg every 2 weeks plus ipilimumab at 1 mg/kg every 6 weeks; or (3) chemotherapy alone.
The co-primary endpoints were overall survival and progression-free survival in the biomarker-defined population with tumor cell PD-L1 expression ≥ 1% (almost half the population). The secondary endpoints were overall survival, progression-free survival, and objective response rate in all randomly assigned patients. The minimum follow-up was 12.9 months.
Survival Outcomes
“In patients with PD-L1 expression ≥ 1%, we observed a highly statistically significant improvement in overall survival, translating into over a 6-month improvement,” Dr. Chau reported. “Also, in the all-randomized population, we observed the same trend favoring nivolumab plus chemotherapy,” he said (Table 1).
In both the primary and secondary populations, he added: “We saw the latter part of the overall survival curve widely separating in favor of nivolumab with chemotherapy or ipilimumab, meaning there were more patients with prolonged overall survival.”
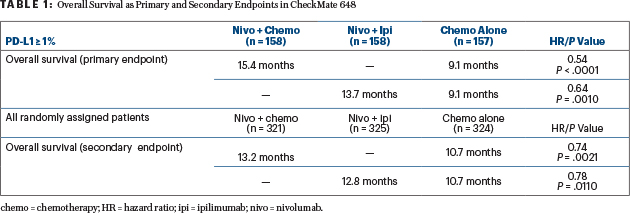
In the primary, PD-L1–positive population, the 12-month overall survival rates were 58% for nivolumab/chemotherapy, 57% for nivolumab/ipilimumab, and 37% for chemotherapy. In the all-randomized population, 12-month survival rates were 54%, 54%, and 44%.
Progression-free survival was significantly improved with nivolumab/chemotherapy in the primary, PD-L1–positive population (hazard ratio [HR] = 0.65; P = .0023), but in the all-randomized population, the benefit was a nonstatistical trend (HR = 0.81). Commenting on the lack of significance in that cohort, Dr. Chau said that many patients received subsequent treatment prior to documentation of disease progression by central review, leading to censoring. A preplanned exploratory analysis showed that by investigator assessment, the benefit of nivolumab/chemotherapy on progression-free survival was greater (HR = 0.69).
A significant progression-free survival benefit was also not seen with nivolumab plus ipilimumab in either population, as expected from other randomized trials of immunotherapy, he said. A late separation of the curves did favor nivolumab/ipilimumab (HR = 0.81), he added.
Objective response rates per blinded independent central review were 53% for nivolumab/chemotherapy, 35% for nivolumab plus ipilimumab, and 20% for chemotherapy, in patients with PD-L1 expression ≥ 1%. In the all-randomized population, response rates were 47%, 28%, and 27%, respectively. With both nivolumab-containing regimens, complete response rates were tripled as compared with chemotherapy alone, and the duration of response was doubled vs chemotherapy.
Safety Profile
As expected, the spectrum of adverse events was different for patients receiving chemotherapy vs dual checkpoint inhibition. With chemotherapy, more nausea, stomatitis, and diminished appetite were seen, whereas with nivolumab/ipilimumab, patients experienced more rash, pruritus, and hypothyroidism.
Serious treatment-related adverse events of grade ≥ 3 were observed in 18% of the nivolumab/chemotherapy arm, 23% of the nivolumab/ipilimumab arm, and 13% of the chemotherapy-alone arm. Treatment-related deaths were similar, with a low incidence in all three groups (1%–2%).
PD-L1 Positivity Not Required
Dr. Chau emphasized that the survival benefit was clear in all patients. Researchers are now evaluating outcomes according to the Combined Positive Score (CPS), which includes staining on immune cells as well as tumor cells. Preliminary data suggest that more than 90% of the CheckMate 648 population has a CPS ≥ 1% expression, which may explain the broad benefit conveyed by immunotherapy.
“In patients across the board, we saw an overall survival advantage. Yes, it’s more enhanced in patients with PD-L1 ≥ 1%…, but I would not say that patients have to be PD-L1–positive to benefit from front-line nivolumab-containing regimens. I would not limit prescribing,” he commented.
Which Regimen for Which Patients?
“We are now fortunate to have two treatment options. When these drugs become available to us, the patient with esophageal squamous cell carcinoma who comes to my clinic will receive immunotherapy. The question is whether to give it with chemotherapy or ipilimumab,” Dr. Chau said.
Consider functional status, comorbidities, and tumor burden, he advised. For the patient with a high symptomatic burden who is fit for chemotherapy, he would opt for nivolumab with chemotherapy to attain a quick response. Fortunately, patients unfit for chemotherapy now have another effective treatment option, he said.
DISCLOSURE: Dr. Chau has received honoraria from Lilly; has served as a consultant or advisor to AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Incyte, Lilly, Merck Serono, MSD Oncology, OncXerna, Pierre Fabre, and Roche/Genentech; has received institutional research funding from Janssen-Cilag and Lilly; and has been reimbursed for travel, accommodations, and other expenses from Bristol Myers Squibb, Lilly, Merck Serono, and MSD.
REFERENCE
1. Chau I, Doki Y, Ajani JA, et al: Nivolumab plus ipilimumab or nivolumab plus chemotherapy versus chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma: First results of the CheckMate 648 study. 2021 ASCO Annual Meeting. Abstract 4001. Presented June 5, 2021.


