In studies reported at the ESMO Congress 2019, immunotherapy yielded encouraging outcomes in two gynecologic cancer populations in need of new treatments, including patients with advanced cervical cancer that is microsatellite-stable and patients previously treated for advanced endometrial cancer.
The combination of nivolumab and ipilimumab, with two different dosing schedules, demonstrated durable clinical activity in recurrent or metastatic cervical cancer in the ongoing open-label phase I/II CheckMate 358 study.1 At a median follow-up of about 1 year, the median overall survival was not reached with nivolumab/ipilimumab in treatment-naive patients.
“The overall survival curves were striking,” said lead investigator Ana Oaknin, MD, PhD, of Vall d’Hebron University Hospital in Barcelona.

Ana Oaknin, MD, PhD

Vicky Makker, MD
In advanced endometrial cancer, the combination of pembrolizumab and the multikinase inhibitor lenvatinib showed “compelling activity” and “demonstrated deep and durable responses,” said Vicky Makker, MD, of Memorial Sloan Kettering Cancer Center, New York, who presented updated findings from a phase II open-label single-arm trial.2
Nivolumab-Based Therapy for Advanced Cervical Cancer
CheckMate 358 is an ongoing phase I/II study that is investigating nivolumab-based therapies in virus-associated cancers, regardless of tumor PD-L1 expression. Patients enrolled were positive for human papillomavirus (HPV), or their HPV status was unknown.
The PD-1 inhibitor pembrolizumab is approved for the treatment of relapsed or metastatic cervical cancer postchemotherapy, but only in patients whose tumors express PD-L1. In CheckMate 358, nivolumab plus ipilimumab showed activity regardless of PD-L1 expression.
In 91 women with squamous cell carcinoma of the cervix who had received no more than two prior systemic therapies for advanced disease, CheckMate 358 evaluated two different regimens combining nivolumab and ipilimumab. The primary endpoint was investigator-assessed objective response rate.
Patients were randomly assigned 1:1 to the following treatment regimens:
- Nivolumab at 3 mg/kg every 2 weeks plus ipilimumab at 1 mg/kg every 6 weeks
- Nivolumab at 1 mg/kg plus ipilimumab at 3 mg/kg, given every 3 weeks for 4 doses, followed by nivolumab at 240 mg every 2 weeks.
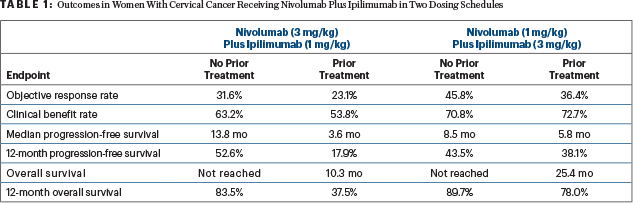
At a median follow-up of 10.7 months in the arm receiving nivolumab at 3 mg/kg plus ipilimumab at 1 mg/kg and 13.9 months in those receiving nivolumab at 1 mg/kg plus ipilimumab at 3 mg/kg, the combinations in both dosing schedules led to high response rates and benefits in progression-free and overall survival (see Table 1). Efficacy was found regardless of the expression of PD-L1.
“Provocatively, across both regimens, efficacy was better in patients without prior systemic therapy,” said Dr. Oaknin. “Additionally, responses were durable. At a median follow-up of more than 10 months, the median duration of response was not reached for either regimen in patients without prior systemic therapy.”
To demonstrate the robust effect of this treatment in some patients, Dr. Oaknin described a 42-year-old woman with HPV-positive recurrent stage IIA disease and lung metastases. After receiving multiple lines of treatment and still experiencing disease progression, she was treated with nivolumab at 3 mg/kg plus ipilimumab at 1 mg/kg and had a complete response at 7.7 months. Biopsy at week 7 showed necrosis alone. She stopped treatment after 2 years and, 7 months later, has no evidence of disease.
“The regimens had a manageable toxicity safety profile, with no new safety signals detected,” she reported. In the study, treatment with [nivolumab at 1 mg/kg plus ipilimumab at 3 mg/kg] led to more treatment discontinuations (33% vs 18%) and more gastrointestinal toxicity (56% vs 36%), which she said may have been due to the higher doses of ipilimumab.
“Given the limited treatment options for patients with recurrent or metastatic cervical cancer,” Dr. Oaknin concluded, “these data with nivolumab plus ipilimumab are of strong clinical interest and warrant further investigation in this patient population.”
Pembrolizumab/Lenvatinib in Endometrial Cancer
Dr. Makker presented an update of the study that led to accelerated approval of the pembrolizumab/lenvatinib regimen in previously treated patients with advanced endometrial cancer with microsatellite-stable tumors—a group that represents about 75% of endometrial cancers. Although PD-1 inhibition is effective in tumors with microsatellite instability–high (MSI-H) or mismatch repair deficiency (dMMR), as a single agent it is less efficacious in patients with microsatellite-stable disease.
In September 2019, the U.S. Food and Drug Administration granted accelerated approval to the combination of lenvatinib and pembrolizumab for the treatment of patients with advanced endometrial carcinoma that is not MSI-H or dMMR who have progressive disease after systemic therapy and who are ineligible for curative surgery or radiation therapy. The approval was based on preliminary findings of the study reported at the ESMO Congress 2019.
OF NOTE
A phase II trial of lenvatinib plus pembrolizumab vs doxorubicin or weekly paclitaxel is ongoing (ClinicalTrials.gov identifier NCT03517449).At data cutoff of January 10, 2019, the analysis included 108 patients who mostly had microsatellite-stable disease and who received this regimen as a second or later line of therapy. The primary endpoint was objective response rate.
By investigator assessment, responses were observed in 38.9% overall, including 37.2% in the cohort without MSI-H/dMMR disease and 63.6% in the small group of patients who did have MSI-H/dMMR tumors. Median progression-free survival was 7.5 months overall, 6.4 months in patients without MSI-H/dMMR tumors, and 18.9 months in those with MSI-H/dMMR tumors. At a median follow-up of 18.7 months, median overall survival was 16.7 months, 18.4 months, and not reached, respectively.
Almost all patients reported a treatment-related adverse event, and 18% discontinued treatment because of toxicity. Two-thirds required dose reductions of lenvatinib; 28% required interruptions of both drugs. The most common treatment-related toxicity was hypertension, which was seen in 60% of patients overall.
Immune-related adverse events were observed in 57% overall, with grade 3 or 4 events in 13%. Aside from a higher incidence of hypothyroidism (43%) than previously reported with either monotherapy, “the safety profile of lenvatinib plus pembrolizumab was similar to previously reported profiles of each monotherapy,” Dr. Makker noted.
DISCLOSURE: Dr. Oaknin has consulted for or received travel funding from AstraZeneca, Clovis Oncology, Genmab, Immunogen, PharmaMar, Roche, and Tesaro. Dr. Makker has served on advisory boards or received honoraria and travel funding from ArQule, Eisai, Karyopharm, Merck Sharp & Dohme, and IBM Watson.
REFERENCES

