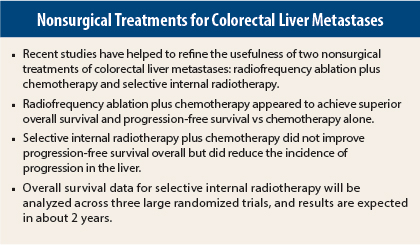
Two “firsts” in studies of colorectal liver metastases were highlighted at the 2015 ASCO Annual Meeting: the first prospective randomized trial to evaluate radiofrequency ablation plus chemotherapy1 and the first large randomized phase III trial to study liver-directed selective internal radiation therapy.2 Radiofrequency ablation plus chemotherapy appeared to have clear benefit in this setting, whereas the results for selective internal radiotherapy in first-line therapy are currently less conclusive. Daniel G. Haller, MD, of Abramson Cancer Center at the University of Pennsylvania, discussed both trials at Best of ASCO® 2015 in Boston.
Radiofrequency Ablation Plus Chemotherapy
Unresectable liver metastases treated with radiofrequency ablation plus chemotherapy improved long-term overall and progression-free survival compared with chemotherapy alone in patients with metastatic colorectal cancer, according to the results of the randomized phase II study of the EORTC NCRI CCSG-ALM Intergroup 40004 (CLOCC).
“This is the first study to prospectively evaluate radiofrequency ablation plus chemotherapy. This study showed that radiofrequency plus chemotherapy is clearly superior to systemic [chemo] therapy in terms of progression-free survival and overall survival in colorectal cancer patients with inoperable liver metastases. This is a no-brainer, and this study is unlikely to ever be repeated,” said Dr. Haller, who reviewed CLOCC and other important studies in gastrointestinal (colorectal) cancer selected for the Best of ASCO 2015.
Between 2002 and 2007, the study enrolled patients with colorectal cancer who had up to nine liver metastases and no extrahepatic disease. A total of 119 patients were randomized to receive chemotherapy alone or radiofrequency ablation plus chemotherapy. In both arms, chemotherapy consisted of 6 months of FOLFOX (leucovorin, fluorouracil, oxaliplatin), and bevacizumab (Avastin) was added in 2005. In both arms, resection was allowed if chemotherapy converted unresectable metastases to resectable.
In the radiofrequency ablation arm, 56 patients were treated with radiofrequency ablation, and 27 patients (45%) underwent hepatic resection. Of the 51 patients in the chemotherapy arm, 6 eventually underwent hepatic resection.
As reported in 2010, 30-month overall survival (primary endpoint) was 61.7% for radiofrequency ablation plus chemotherapy and 57.6% for chemotherapy alone, which was higher than expected.
At a median follow-up of 9.2 years, the difference in overall survival significantly favored the radiofrequency ablation arm (P = .01). Median overall survival was 45.6 months in the radiofrequency ablation arm vs 40.5 months in the chemotherapy-alone arm. Eight-year overall survival was 35.9% vs 8.9%, respectively (P = .010). A total of 92 deaths were reported: 39 in the radiofrequency ablation arm and 53 in the chemotherapy-alone arm. Progressive disease accounted for 34 and 48 deaths, respectively.
Progression-free survival was significantly superior in the radiofrequency ablation arm. With long-term follow-up, median progression-free survival was 16.8 months for radiofrequency ablation vs 9.9 months for chemotherapy alone (P = .025). Eight-year progression-free survival was 22% vs 2%, respectively (P = .005).
SIRFLOX Study
In the randomized, phase III SIRFLOX study, first-line therapy for patients with unresectable liver metastases with selective internal radiotherapy added to modified FOLFOX6 plus or minus bevacizumab failed to improve overall progression-free survival compared with modified FOLFOX6 plus or minus bevacizumab (which was not part of the randomization scheme) did better than those who did not.
Selective internal radiotherapy employs yttrium-90–labelled resin microspheres or glass beads as a liver-directed therapy, and it has been approved by the U.S. Food and Drug Administration for the treatment of hepatic cell carcinoma, neuroendocrine tumors, and metastatic colorectal cancer.
SIRFLOX randomized 530 patients with nonresectable liver-only or liver-dominant metastatic colorectal cancer with no prior therapy in a 1:1 ratio to receive treatment with modified FOLFOX6 plus or minus bevacizumab or modified FOLFOX6 plus selective internal radiotherapy plus or minus bevacizumab. Patients were prestratified according to extrahepatic metastasis, degree of liver involvement, and intended use of bevacizumab.
Inclusion criteria allowed extrahepatic metastases as follows: up to five lung metastases smaller than 1 cm and lymph nodes in a single anatomic region. Less than 40% of patients had extrahepatic disease. About 50% of patients received bevacizumab.
For the primary endpoint of overall progression-free survival, there was no significant difference between the two treatment arms. “However, progression-free survival in the liver (a secondary endpoint) had interesting results,” Dr. Haller noted.
Median progression-free survival in the liver was 12.6 months for chemotherapy vs 20.5 months for selective internal radiotherapy (P = .002), representing a 7.9-month improvement and a 31% risk reduction for disease progression in the liver. Complete response in the liver was achieved in 2% of the chemotherapy arm vs 6% in the selective internal radiotherapy arm (P = .020).
“Selective internal radiotherapy did not enable more liver resections and does not convert patients or downstage them,” Dr. Haller said.
More adverse events occurred with patients treated with selective internal radiotherapy. The rate of any adverse event was 73.4% for chemotherapy and 85.4% for selective internal radiotherapy. Grade 3 or higher adverse events follow: neutropenia, 28.5% for chemotherapy vs 40.7% for selective internal radiotherapy; febrile neutropenia, 1.9% vs 6.1%; thrombocytopenia, 2.6% vs 9.8%.
Future analysis of these data will include liver-only vs liver-dominant outcomes, depth of response, quality of life, cost-effectiveness of a single treatment of selective internal radiotherapy vs systemic treatment, and overall survival.
“Selective internal radiotherapy achieved an 8-month improvement in progression-free survival in the liver, but it is not clear what this means for the lifespan of the patient. Selective internal radiotherapy did not add much to the overall response rate, but it was superior in the liver,” he continued. “However, toxicities are noteworthy.”
SIRFLOX is the first of three studies to look at a pooled analysis of overall survival; the other two ongoing trials are FOXFIRE and FOXFIRE global. Overall survival results of all three trials, for a total of 2,017 patients, should be available in about 2 years.
Additional Discussion
“There are multiple options for treatment of metastatic disease to the liver. They include systemic therapy, surgery, neoadjuvant therapy, liver-directed therapy, selective internal radiotherapy [SIRFLOX], and radiofrequency ablation [CLOCC],” Dr. Haller said. “The goal of these latter treatments is to allow patients to be resected, delay biliary obstruction, [and] maintain liver function, so patients can get later lines of therapy. The timing is questionable, but I believe in the middle of chemotherapy is optimal,” he said.
“[The phase II Intergroup 40004] study included patients with unresectable liver metastasis. Patients were allowed chemotherapy prior to randomization. The study had a huge bias in that there were more patients with a single liver metastasis in the radiofrequency ablation arm. In addition, about 50% of patients treated with radiofrequency ablation had resection, making it difficult to distinguish which patients were helped by radiofrequency ablation. Also, there were small numbers of patients, and they were highly selected,” Dr. Haller continued.
“Selective internal radiotherapy bought patients an extra 8 months of progression-free survival in the liver, but this therapy has toxicities. Analysis of overall survival and the impact of the initial large increase in liver progression-free survival on further lines of therapy will add more data on this modality,” concluded Dr. Haller. ■
Disclosure: Dr. Haller is on the advisory board of Sirtex.
References
1. Ruers T, Punt CJA, van Coevorden F, et al: Radiofrequency ablation combined with chemotherapy for unresectable colorectal liver metastases: Long-term survival results of a randomized phase II study of the EORTC-NCRI CCSG-ALM Intergroup 40004 (CLOCC). 2015 ASCO Annual Meeting. Abstract 3501. Presented May 29, 2015.
2. Gibbs P, Heinemann V, Sharma NK, et al: SIRFLOX: Randomized phase III trial comparing first-line mFOLFOX6 ± bevacizumab versus mFOLFOX6 ± selective internal radiation therapy ± bevacizumab in patients with metastatic colorectal cancer. 2015 ASCO Annual Meeting. Abstract 3502. Presented May 29, 2015.