The Cancer and Leukemia Group B (CALGB)/Southwest Oncology Group (SWOG) 80405 trial, presented during the Plenary Session at this year’s ASCO Annual Meeting, demonstrated that cetuximab (Erbitux) and bevacizumab (Avastin) confer similar benefits as first-line treatment with chemotherapy for KRAS wild-type metastatic colorectal cancer.1
The ASCO Post recently spoke to Alok A. Khorana, MD, Professor of Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University; and Sondra and Stephen Hardis Chair in Oncology Research, Vice Chair of Clinical Services, and Director of the GI Malignancies Program at Taussig Cancer Institute, Cleveland Clinic, about the study findings and its implications for clinical practice, as well as other important data in colorectal cancer presented at the Annual Meeting
Groundbreaking Abstract
What’s your take on the CALGB/SWOG 80405 study?
CALGB/SWOG 80405 had to be the most groundbreaking abstract on colorectal cancer presented at this year’s ASCO Annual Meeting, as reflected by its selection for the Plenary Session. The study took 10 years to complete and asked the question, “What’s the most appropriate first-line treatment in metastatic colorectal cancer?” The reason it took 10 years to complete is that in the first couple of years, new findings suggested that one of the arms should be dropped, so there was an amendment. Additional new findings a couple of years later suggested that only KRAS wild-type patients should be included, so there was a second amendment.
The study underwent several iterations before it arrived at the final design. In the end, it was a two-arm study of a chemotherapy backbone, which was up to the physician, and random assignment to either cetuximab, an epidermal growth factor receptor (EGFR) inhibitor, or bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor. It’s billed as a “negative” study because there was no difference in outcomes between the two arms, but it does have a lot of relevance to clinical practice.
We’ve always wondered about the most appropriate first-line treatment in this setting, and the answer for right now is that it could be either one of these agents. The one downside of cetuximab is that there is increased skin toxicity, which was shown in this study as well. However, the skin toxicity did not seem to affect quality of life; the quality-of-life questionnaires were comparable between the two arms.
The second bit of good news from the study is that the median survival of cancer patients in this study was 29 months—that’s 2½ years. If you go back to the 1990s, the median survival was 12 months, and if you go back to the past decade, it was 20 or 22 months. So 29 months is a phenomenal improvement in median survival for patients with metastatic disease, and I think that is really something to be proud of for the gastrointestinal cancer community.
A third takeaway from CALGB/SWOG 80405 is that there are still findings to be made from this particular clinical trial. In just the past 6 months, we’ve discovered that there are additional RAS mutations that might limit the benefit of cetuximab. Subgroup analysis is underway to find those RAS mutations in this population and to exclude those patients from the general effects observed in the study. That subgroup analysis may yet alter the conclusions of the trial.
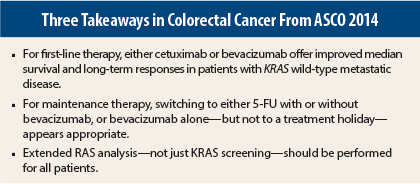
So for now, you can use either cetuximab or bevacizumab in first-line treatment of KRAS wild-type metastatic colorectal cancer. Toxicities appear to be similar, median survival is close to 2½ years, and at 5 years, one-tenth of patients—almost 11% of the patients in this study—do not have cancer with metastatic disease.
Maintenance Chemotherapy
What do the new data presented at the Annual Meeting show with regard to the benefit of maintenance chemotherapy?
Once you get to about 6 months of therapy, most patients have too much drug-related toxicity and want to scale back their treatment. What options should be chosen at that time? Currently available options include the use of treatment holidays, in which you stop using drug therapy and follow those patients. Alternatively, they can continue on aggressive chemotherapy with more treatment combinations, or scale down chemotherapy to just one or two drugs with the biologic.
Multiple abstracts have dealt with these options, and they all point in the same direction: treatment holidays are probably not the best route.2,3 The type of maintenance therapy could be either fluorouracil (5-FU) or capecitabine with bevacizumab, or it could be bevacizumab alone. The three approaches were not statistically significantly different, but if you look at the absolute benefit, there is a clear benefit for the two-drug combination of either 5-FU/bevacizumab or capecitabine/bevacizumab. For most patients, that is a gentler regimen.
So now that we’ve sorted out the first-line treatment, the maintenance portion is likely not treatment holiday but a combination of a 5-FU–based regimen with an antibody, probably bevacizumab.
Extended RAS Testing
What do the data show on the use of extended RAS testing?
We’ve seen reports in the past couple of months looking at extended RAS testing, and up to 20% of patients—in one study, up to 30%—who we thought had wild-type disease and would benefit from an anti-EGFR approach, are no longer wild-type.4,5 Yet these patients have been getting an anti-EGFR antibody, they have been experiencing the skin toxicity, and the costs to the system have been very expensive.
Data presented at this meeting, particularly the subgroup analysis of the OPUS6 and CRYSTAL7 large clinical trials all show that these patients do not show benefit from anti-EGFR therapy, so the new standard of care should be that all metastatic colorectal cancer patients should be screened, not just for KRAS, which has been our standard for close to 10 years, but also with an extended RAS analysis, which includes NRAS and other codons of KRAS. ■
Disclosure: Dr. Khorana is a consultant for and receives honoraria from Sanofi and Genentech.
References
1. Venook AP, Niedzwiecki D, Lenz H-J, et al: CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab or cetuximab for patients with KRAS wild-type untreated metastatic adenocarcinoma of the colon or rectum. ASCO Annual Meeting. LBA3. Presented June 1, 2014.
2. Arnold D, Graeven U, Lerchenmuller CA, et al: Maintenance strategy with fluoropyrimidines (FP) plus bevacizumab (Bev), Bev alone, or no treatment, following a standard combination of FP, oxaliplatin (Ox), and Bev as first-line treatment for patients with metastatic colorectal cancer (mCRC): A phase III non-inferiority trial (AIO KRK 0207). ASCO Annual Meeting. Abstract 3503. Presented June 2, 2014.
3. Koopman M, Simkens L, May AM, et al: Final results and subgroup analyses of the phase 3 CAIRO3 study: Maintenance treatment with capecitabine + bevacizumab versus observation after induction treatment with chemotherapy + bevacizumab in metastatic colorectal cancer. ASCO Annual Meeting. Abstract 3504. Presented June 2, 2014.
4. Tejpar S, Lenz HJ, Köhne CH, et al: Effect of KRAS and NRAS mutations on treatment outcomes in patients with metastatic colorectal cancer (mCRC) treated first-line with cetuximab plus FOLFOX4: New results from the OPUS study. 2014 Gastrointestinal Cancers Symposium. Abstract LBA444. Presented January 16, 2014.
5. Stintzing S, Jung A, Rossius L, et al: Analysis of KRAS/NRAS and BRAF mutations in FIRE-3: A randomized phase III study of FOLFIRI plus cetuximab or bevacizumab as first-line treatment for wild-type (WT) KRAS (exon 2) metastatic colorectal cancer (mCRC) patients. 2013 European Cancer Congress. Abstract LBA17. Presented September 28, 2013.
6. Bokemeyer C, Kohne CH, Ciardiello F, et al: Treatment outcome according to tumor RAS mutation status in OPUS study patients with metastatic colorectal cancer (mCRC) randomized to FOLFOX4 with/without cetuximab. ASCO Annual Meeting. Abstract 3505. Presented June 2, 2014.
7. Ciardiello F, Lenz HJ, Kohne CH, et al: Treatment outcome according to tumor RAS mutation status in CRYSTAL study patients with metastatic colorectal cancer (mCRC) randomized to FOLFIRI with/without cetuximab. ASCO Annual Meeting. Abstract 3506. Presented June 2, 2014.