In KEYNOTE-177, the anti–PD-L1 antibody pembrolizumab reduced the risk of disease progression by 40% vs chemotherapy in a targeted subset of previously untreated patients with metastatic colorectal cancer. Now, in terms of health-related quality of life, pembrolizumab is also the clear favorite, based on the results of this prespecified exploratory endpoint reported at the European Society for Medical Oncology (ESMO) Virtual Congress 2020.1
“The health-related quality-of-life results complement the superior progression-free survival and lower treatment-related adverse event rate with pembrolizumab vs chemotherapy with or without bevacizumab or cetuximab in KEYNOTE-177,” said the study’s principal investigator Thierry André, MD, of Sorbonne University and Saint-Antoine Hospital, Paris.

“The health-related quality-of-life results complement the superior progression-free survival and lower treatment-related adverse event rate with pembrolizumab….”— Thierry André, MD
Tweet this quote
The phase III KEYNOTE-177 trial showed, for the first time, that upfront treatment with immunotherapy (pembrolizumab) could improve progression-free survival vs chemotherapy as a first-line treatment of microsatellite instability–high (MSI-H)/mismatch repair–deficient (dMMR) metastatic colorectal cancer, Dr. André reported in a Plenary Session presented during the ASCO20 Virtual Scientific Program.2
KEYNOTE-177 Details
The open-label study randomly assigned 307 previously untreated patients to first-line pembrolizumab at 200 mg every 3 weeks for up to 2 years or investigator’s choice of modified FOLFOX6 (fluorouracil [5-FU], leucovorin, oxaliplatin) or FOLFIRI (5-FU, leucovorin, irinotecan) every 2 weeks, with or without bevacizumab or cetuximab.
Patients receiving pembrolizumab had a median progression-free survival of 16.5 months vs 8.2 months with chemotherapy (hazard ratio [HR] = 0.60; P = .0002), and thus, KEYNOTE-177 met one of its two co-primary endpoints (overall survival will be reported later). “The study was considered successful if pembrolizumab was superior to chemotherapy for either primary endpoint,” Dr. André said.
Quality-of-Life Findings
Patent-reported outcomes constituted a prespecified exploratory endpoint. These parameters included mean score change from baseline to week 18 in the following scales and items: European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 (QLQ-C30), EORTC QLQ-CR29, and EuroQoL 5 Dimensions 3 Levels (EQ-5D-3L). Altogether, these instruments comprise a variety of functional scales in physical, social, cognitive, and emotional domains, measuring symptoms, mobility, self-care, usual activity, pain and discomfort, anxiety and depression, and some colorectal cancer–specific factors. Time to deterioration in the EORTC QLQ-C30 was another patient-reported outcome. Data were collected at baseline, during treatment, espescially at week 18, and 30 days after treatment discontinuation.
The EORTC QLQ-C30 questionnaire was completed at baseline by 93% of each group. It was again completed at the prespecified week 18 time point by 88% of the pembrolizumab arm and 77% of the chemotherapy arm and at week 45 by 84% and 56%, respectively. Similar compliance was observed for the other quality-of-life instruments.
KEY POINTS
- KEYNOTE-177 found that first-line pembrolizumab significantly improved progression-free survival vs chemotherapy in patients with metastatic colorectal cancer with MSI-H/dMMR tumors.
- In the prespecified exploratory analysis of patient-reported outcomes, pembrolizumab was also associated with significantly better health-related quality-of-life scores.
- Scores generally improved in patients receiving pembrolizumab but worsened for those treated with chemotherapy.
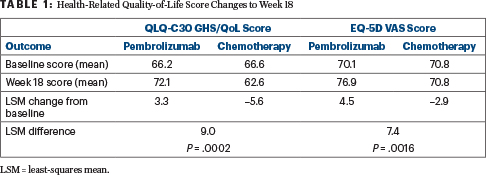
“The EORTC QLQ-C30, GHS/QoL, and EQ-5D visual analog scale scores were similar at baseline between the groups. At week 18, the QLQ-C30 improved over baseline in the pembrolizumab arm but worsened in the chemotherapy group. Least squares mean (LSM) change from baseline to week 18 showed improvement in QLQ-C30 GHS/QoL with pembrolizumab versus chemotherapy (LSM difference: 8.96; 95% CI 4.24 to 13.69; P = .0002).
In the Colo-rectal cancer EQ-5D scale, scores also improved with pembrolizumab and worsened with chemotherapy,” Dr. André reported
“From baseline to week 18, patients on pembrolizumab generally exhibited stable or improved scores in most of the QLQ-C30 domains and improved scores in most of the symptom domains, whereas patients in the chemotherapy arm generally exhibited worsening scores for all except the emotional function domain,” he said.
The domains showing the largest differences between the treatments, favoring pembrolizumab, were physical function, role function, social function, fatigue, pain, and appetite loss. In many domains, scores continued to improve out to week 45 for patients receiving pembrolizumab, but worsened over time in those treated with chemotherapy. Pembrolizumab also significantly prolonged the time to deterioration in physical functioning, social functioning, and fatigue, he added.
The EORTC QLQ-C29 scale assesses cancer–associated functioning and symptoms. As for the other scales, the pembrolizumab group “generally showed stable or improved scores in most functional scales except anxiety, which improved with both treatments,” Dr. André said.
DISCLOSURE: KEYNOTE-177 was funded by Merck Serono and Pfizer. Dr. André has received honoraria from Amgen, Bristol Myers Squibb, GlaxoSmithKline, Roche/Genentech, Pierre Fabre, Sanofi, SERVIER, and Ventana; has served in a consulting or advisory role for Amgen, AstraZeneca/MedImmune, Bristol Myers Squibb, Clovis Oncology, Gamamabs pharma SA, GIC Advice, HalioDx, MSD Oncology, Pierre Fabre, Servier, and Tesaro; and has been reimbursed for travel, accommodations, or other expenses by Amgen, Bristol Myers Squibb, MSD Oncology, Roche, Roche/Genentech, and Ventana.
REFERENCES
1. André T, Amonkar M, Norquist J, et al: Health-related quality of life in patients treated with pembrolizumab vs chemotherapy as first-line treatment in microsatellite instability-high and/or deficient mismatch repair metastatic colorectal cancer: Phase III KEYNOTE-177 study. ESMO Virtual Congress 2020. Abstract 396O. Presented September 19, 2020.
2. André T, Shiu KK, Kim TW, et al: Pembrolizumab vs chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: The phase III KEYNOTE-177 study. ASCO20 Virtual Scientific Program. Abstract LBA4.

