In the Clinic provides overviews of novel oncology agents, addressing indications, mechanisms, administration recommendations, safety profiles, and other essential information needed for the appropriate clinical use of these drugs.
On September 30, 2015, the U.S. Food and Drug Administration granted accelerated approval to nivolumab (Opdivo) in combination with ipilimumab (Yervoy) for the treatment of patients with BRAF V600 wild-type unresectable or metastatic melanoma, including those with previously untreated disease.1,2
Supporting Efficacy Data
Approval was based on demonstration of improved objective response rate, prolonged response duration, and improved progression-free survival in an international, multicenter, double-blind phase II trial in 142 previously untreated patients.3 Patients were randomly assigned 2:1 to receive intravenous nivolumab at 1 mg/kg and ipilimumab at 3 mg/kg every 3 weeks for four doses and then nivolumab at 3 mg/kg every 2 weeks (n = 95, including 72 with wild-type BRAF V600) or intravenous ipilimumab at 3 mg/kg and placebo every 3 weeks for four doses followed by intravenous placebo every 2 weeks (n = 47, including 37 with wild-type BRAF V600) until disease progression or unacceptable toxicity.
Among the 109 patients with BRAF V600 wild-type disease, median age was 66 years, Eastern Cooperative Oncology Group performance score was 0 in 84% and 1 in 15%, 46% had M1c disease, and 20% had elevated lactate dehydrogenase. Baseline differences between the combination and ipilimumab groups consisted of history of brain metastasis (6% vs 0%), acral/mucosal melanoma (10% vs 24%), and cutaneous melanoma (82% vs 57%).
Among patients with wild-type BRAF V600, the objective response rate was 60% in the combination group vs 11% in the ipilimumab group (P < .001). Median progression-free survival was 8.9 months (95% confidence interval [CI] = 7.0 months to not estimable) vs 4.7 months (95% CI = 2.8–5.3 months; hazard ratio = 0.40, P < .002). Of the 43 patients with objective response in the nivolumab-plus-ipilimumab group, 9 (21%) had disease progression after responses of 3 to 7 months, and 34 (79%) had ongoing responses at the time of last analysis (6 to < 9 months in 14 and ≥ 9 months in 20).
How It Works
Nivolumab is a human immunoglobulin G4 monoclonal antibody that binds the programmed cell death protein (PD-1) receptor on T cells and prevents its interaction with the ligands PD-L1 and PD-L2, thereby blocking PD-1 pathway–mediated inhibition of immune response, including antitumor immune response. Binding of PD-L1 and PD-L2 to the PD-1 receptor inhibits T-cell proliferation and cytokine production.
Upregulation of PD-1 ligands occurs in some tumors, and signaling through this pathway can contribute to inhibition of active T-cell tumor immune surveillance. In syngeneic mouse tumor models, blocking PD-1 activity results in decreased tumor growth.
Combined PD-1 inhibition with nivolumab and CTLA-4 inhibition with ipilimumab produces enhancement of T-cell function that is greater than with either agent alone, resulting in improved antitumor responses in metastatic melanoma. In murine syngeneic tumor models, dual blockade of PD-1 and CTLA-4 resulted in increased antitumor activity.
How It Is Used
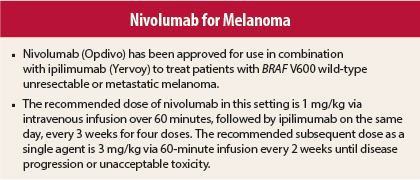
The recommended dose of nivolumab in this setting is 1 mg/kg via intravenous infusion over 60 minutes, followed by ipilimumab on the same day, every 3 weeks for four doses. The recommended subsequent dose as a single agent is 3 mg/kg via 60-minute infusion every 2 weeks until disease progression or unacceptable toxicity.
Nivolumab treatment should be withheld for grade 2 pneumonitis, grade 2 or (when used as a single agent) grade 3 diarrhea or colitis, aspartate aminotransferase (AST) or alanine aminotransferase (ALT) > 3 to 5 times the upper limit of normal or total bilirubin > 1.5 to 3 times the upper limit of normal, serum creatinine > 1.5 to 6 times the upper limit of normal or > 1.5 times baseline level, grade 2 or 3 hypophysitis, grade 2 adrenal insufficiency, grade 3 rash, and first occurrence of other grade 3 adverse reactions.
There are no recommended dose modifications for hypothyroidism or hyperthyroidism. No dose adjustment is required in patients with renal impairment or mild hepatic impairment; the drug has not been studied in patients with moderate or severe hepatic impairment. Ipilimumab should be withheld when nivolumab is withheld.
Nivolumab should be discontinued for grade 3 diarrhea or colitis with combined use, grade 4 hypophysitis, grade 3 or 4 adrenal insufficiency, grade 4 rash, any life-threatening or grade 4 adverse reaction, grade 3 or 4 pneumonitis, grade 4 colitis, AST or ALT > 5 times the upper limit of normal or total bilirubin > 3 times the upper limit of normal, serum creatinine > 6 times the upper limit of normal, any recurring severe or grade 3 treatment-related adverse reaction, inability to reduce corticosteroid dose to ≤ 10 mg/d of prednisone or equivalent within 12 weeks, and persistent grade 2 or 3 treatment-related adverse reactions that do not recover to grade 0 or 1 within 12 weeks after the last dose.
Infusion should be interrupted or slowed in patients with mild or moderate infusion reactions. Nivolumab should be discontinued for severe or life-threatening infusion reactions.
Safety Profile
Among 140 patients in the phase II trial who received at least one dose of study drug, the most common adverse events of any grade occurring more frequently in the combination group were rash (67% vs 57%), pruritus (37% vs 26%), headache (24% vs 20%), vomiting (23% vs 15%), and colitis (22% vs 11%). Grade 3 or 4 adverse events occurred in 69% of the combination group vs 43% of the ipilimumab group; the most common in the combination group that occurred more frequently than in the ipilimumab group was dehydration (3.2% vs 2.2%).
The most common laboratory abnormalities of any grade occurring more frequently in the combination group were increased ALT (45% vs 20%), increased AST (43% vs 22%), anemia (40% vs 35%), hyponatremia (38% vs 20%), lymphopenia (37% vs 30%), increased lipase (36% vs 17%), and increased alkaline phosphatase (30% vs 17%). The most common grade 3 or 4 abnormalities occurring more frequently in the combination group were increased ALT (13% vs 0%), increased lipase (13% vs 7%), increased AST (10% vs 0%), hyponatremia (9% vs 2%), and lymphopenia (9% vs 2%).
Serious adverse events occurred in 62% vs 39% of patients, with the most common in the combination group being colitis (17% vs 9%), diarrhea (9% vs 7%), pyrexia (6% vs 7%), and pneumonitis (5% vs 0%). Adverse events led to dose delay in 47% vs 22% and permanent discontinuation in 43% vs 11%. The most common causes of discontinuation were colitis (16% vs 2%), diarrhea not treated with corticosteroids (4% vs 4%), increased ALT (4% vs 0%), pneumonitis (3% vs 0%), and increased AST (3% vs 0%).
Among immune-related adverse events in the combination group, pneumonitis occurred in 6% of patients, colitis in 33%, hepatitis in 15%, hypophysitis in 13%, adrenal insufficiency in 9%, hypothyroidism in 19%, hyperthyroidism in 2.1%, nephritis or renal dysfunction in 2.1%, and rash in 37%. Guillain-Barré syndrome and hypopituitarism were observed in 1%.
Nivolumab carries warnings/precautions for immune-mediated adverse reactions, including immune-mediated pneumonitis, colitis, hepatitis, nephritis and renal dysfunction, and hypothyroidism and hyperthyroidism, and embryofetal toxicity. Patients should be monitored for liver, kidney, and thyroid function. Breastfeeding women should discontinue breastfeeding.
Other causes should be excluded for suspected immune-mediated adverse reactions. Based on the severity of the adverse reaction, nivolumab should be withheld or discontinued, and high-dose corticosteroids and, if appropriate, thyroid hormone replacement should be given. Upon improvement of adverse reactions to no worse than grade 1, corticosteroid taper should be started and continued over at least 1 month. Restarting of nivolumab can be considered after completion of the corticosteroid taper. ■
References
1. U.S. Food and Drug Administration: Nivolumab in combination with ipilimumab. Available at www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm465274.htm. Accessed October 7, 2015.
2. Opdivo (nivolumab) injection for intravenous use prescribing information, Bristol-Myers Squibb Company, September 2015. Available at www.accessdata.fda.gov/drugsatfda_docs/label/2015/125554s002lbl.pdf. Accessed October 7, 2015.
3. Postow MA, Chesney J, Pavlick AC, et al: Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372:2006-2017, 2015.
Report Adverse Events
Health-care professionals should report all serious adverse events suspected to be associated with the use of any medicine or device to FDA’s MedWatch Reporting System by completing a form online at www.fda.gov/medwatch/report.htm, by faxing (800-FDA-0178), by mailing the postage-paid address form provided online, or by telephone (800-FDA-1088).