In the neoadjuvant treatment of breast cancer, the importance of achieving a pathologic complete response (pCR) varies substantially by breast cancer subtype. Patients are increasingly interested in this outcome, but it means different things to different patients, according to two breast cancer specialists from Emory University, who discussed the meaning of pCR at the Debates and Didactics in Hematology and Oncology meeting in Sea Island, Georgia.
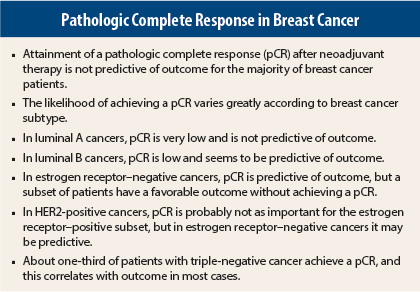
“It’s complicated, but pathologic complete response is not predictive of outcome for the majority of breast cancers,” said Ruth M. O’Regan, MD, Professor of Hematology and Medical Oncology at Emory University and Chief of Hematology and Medical Oncology at the Georgia Cancer Center for Excellence at Grady Memorial Hospital, Atlanta.
Amelia B. Zelnak, MD, Assistant Professor of Hematology and Medical Oncology at Emory, added, “As we use more and more neoadjuvant therapy, our patients are really attuned to how the treatment is working. We need to be careful about how we discuss this with them. There are patients who achieve a pCR who still have recurrences, and many others who don’t achieve a pCR and still do well
long-term.”
Dr. Zelnak noted that the early preoperative trials—especially National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and B-27—showed that patients achieving a pathologic complete response had better long-term outcomes, but these early data were not broken down by HER2 or hormone receptor status, and were conducted before intrinsic subtyping entered the picture.
Since that time, the issue of pathologic complete response—who achieves it, what it means—has become infinitely more nuanced.
Likelihood of Achieving a pCR
The recent meta-analysis by Cortazar et al illustrates the huge variability in pathologic complete response by breast cancer subtype.1 In general, hormone receptor–positive cancers (luminal A and B) have the least chance of a pCR after neoadjuvant chemotherapy, especially if they are low-grade. HER2-positive cancers have a better chance, especially the hormone receptor–negative subset, and approximately one-third of patients with triple-negative breast cancer achieve pCR.
“The point is, if we really believe that pCR is the gold standard for outcome, we have a lot of patients who, unfortunately, don’t achieve it,” Dr. O’Regan commented.
Estrogen receptor–positive cancers are the least responsive to neoadjuvant chemotherapy and need alternative treatments in the preoperative setting. “We are struggling with how to downstage these cancers,” Dr. O’Regan added.
She and Dr. Zelnak compared chemotherapy to endocrine neoadjuvant therapy in a pilot study of 46 patients stratified by 21-gene recurrence score.2 Patients with a recurrence score ≥ 25 received docetaxel plus cyclophosphamide, while those with recurrence score ≤ 10 received exemestane; patients with recurrence score ranging from 11 to 24 were randomly assigned between these arms. The only patients to achieve a pCR (22%) were those in the highest recurrence score group who received chemotherapy. Chemotherapy was also associated with a high rate of complete and partial radiologic responses (90%) in the intermediate-risk group; the response rate was lower with exemestane (67%).
The fact that two-thirds or more of patients did respond indicates that downstaging is occurring. However, 80% of patients did not achieve a pathologic complete response, she emphasized. “If pCR is important in estrogen receptor–positive cancer, these patients need a different approach,” she suggested.
Meta-analysis Important, but Perhaps Misleading
Cortazar’s meta-analysis of almost 12,000 patients in a dozen neoadjuvant studies concluded that patients who attain a pathologic complete response have improved event-free survival.1 With the attainment of pCR, defined by ypT0/is ypN0 (ie, absence of invasive cancer in the breast or nodes; ductal carcinoma in situ allowed), events were reduced by 52% (hazard ratio [HR] = 0.48). However, some associations between pCR and long-term outcomes were far stronger than others. Hazard ratios for event-free survival were greatest for patients with triple-negative cancers (HR = 0.24), of whom 34% achieved a pathologic complete response, and for patients with HER2-positive, hormone receptor–negative disease receiving trastuzumab (Herceptin, HR = 0.25). In estrogen receptor–positive tumors, grade was also important. The pCR rate for low-grade tumors was only 7%, whereas it was 16% for grade 3 tumors.
The German Breast Group also looked in detail at pCR and disease-free survival by intrinsic subgroup and showed no impact of pCR in the luminal A group or among luminal B cancers that are HER2-positive.3 The achievement of pCR was predictive in the three other subsets: luminal B HER2-negative, HER2-positive/estrogen receptor–negative, and triple-negative.
“Overall, pCR is a surrogate marker for outcome in breast cancer but this varies according to the breast cancer subtype. In particular, pCR is not a good marker in low-grade [estrogen receptor]–positive disease,” Dr. O’Regan emphasized.
However, Dr. Zelnak added, “[In our pilot trial] even though pCR rates are much lower among hormone receptor–positive patients, it still appears that patients who achieve a pCR do better long-term.… It still has some prognostic value among HER2-positive, hormone receptor–positive cancers.”
Pertuzumab and pCR
The U.S. Food and Drug Administration (FDA) used the findings from Cortazar’s meta-analysis to support the approval of pertuzumab (Perjeta) in the neoadjuvant setting. The neoadjuvant NeoSphere trial had shown a 46% pathologic complete response rate among patients receiving pertuzumab in addition to docetaxel and trastuzumab,4 and in TRYPHAENA, pertuzumab plus trastuzumab given with chemotherapy produced pCRs in 81% of hormone receptor–negative patients and 47% of hormone receptor–positive ones.5 Approval of the drug was further bolstered by data from the metastatic setting in CLEOPATRA, where pertuzumab added to trastuzumab and docetaxel improved not only response rates and progression-free survival, but also overall survival (HR = 0.66, P = .0008),6 Dr. Zelnak noted.
The adjuvant APHINITY trial should elucidate the association between pathologic complete response and long-term outcomes. “We are optimistic that APHINITY will confirm long-term improvements in outcomes with pertuzumab,” Dr. Zelnak said. “However, we felt more comfortable with this before we saw the data from ALTTO.”
NeoALTTO vs ALTTO
“For HER2-positive disease, we think the truth is in the big trials,” Dr. O’Regan said. For this reason, ALTTO was met with disappointment.
In NeoALTTO, the addition of lapatinib (Tykerb) to trastuzumab resulted in a pathologic complete response rate of 51%, vs 30% with trastuzumab alone and 25% with lapatinib; the highest pCR rate, 61%, was obtained with dual blockade among the hormone receptor–negative subset.7 At 3 years, events were reduced by 62% among patients achieving a pCR vs not (P = .0003), and survival was also significantly improved (P = .005).
“The suggestion, again, was that pCR is more predictive of outcome in hormone receptor–negative cancers, and these patients may do better with the combination of two anti-HER2 agents,” Dr. O’Regan observed. “This was very exciting. We were hopeful it would translate into a meaningful difference in the adjuvant setting.”
ALTTO, however, failed to confirm the benefit of dual anti-HER2 blockade, which Dr. O’Regan attributed, in part, to the population being at lower risk (40% node-negative, 60% hormone receptor–positive) than those in NeoALTTO or in the pivotal adjuvant trastuzumab trials.8 The fact that the overall 4-year disease-free survival rate approached 90% in all arms indicated these were relatively good-prognosis patients, she added.
“NeoALTTO did not translate into differences in the adjuvant setting, for whatever reason, and this begs the question of whether we rushed into using pCR as a surrogate for long-term outcomes.… I think we should have been more reticent about hormone receptor status,” Dr. O’Regan suggested.
Dr. Zelnak indicated that the FDA considered “the body of evidence” in the case of pertuzumab, and rather than universally embrace pCR as a surrogate endpoint for long-term outcomes, the FDA is more likely to evaluate drugs on a case-by-case basis.
Heterogeneity of HER2-Positive Disease
HER2-positive breast cancer demonstrates marked heterogeneity, and this may be influencing pCR rates, Dr. O’Regan indicated.
Carey et al recently reported that in hormone receptor–positive tumors, nearly 50% are luminal B, 34% are luminal A, and 17% are the HER2 intrinsic subtype.9 Luminal A tumors do not derive much benefit from chemotherapy, and pCRs in this subgroup are not very predictive of outcomes. “Being one-third of HER2-positive, hormone receptor–positive tumors, are the luminal A tumors driving pCR rates down in this group?” Dr. O’Regan asked.
The influence of estrogen receptor within HER2-positive tumors has been explored by University of Pittsburgh researchers, who showed that pathologic complete response rates following trastuzumab therapy are highest (52%) in cancers that are hormone receptor–negative, next highest in cancers with weak-to-moderate estrogen receptor expression (33%), and lowest in HER2-positive cancers with the strongest estrogen receptor expression (8%).10 They concluded that the addition of trastuzumab to neoadjuvant chemotherapy significantly increases pCR rates in all HER2-positive tumors, but the benefit of trastuzumab and chemotherapy decreases as estrogen receptor expression increases.
“These findings suggest that the triple-positive cancers perhaps need to be treated differently,” she said, “and there may be many who can get by with less treatment.”
Triple-Negative Disease
Dr. O’Regan and Dr. Zelnak emphasized that pathologic complete response is clearly important in triple-negative disease, and it may also vary according to the subtype within triple-negative disease. (Six subtypes have been described.)
An emerging question, according to Dr. Zelnak, is whether carboplatin should be added to the neoadjuvant regimens for triple-negative tumors. In CALGB 40603, the addition of carboplatin to paclitaxel produced a 60% pCR rate, vs 46% without carboplatin,—an increase of 76% with carboplatin on board (P = .0018).11 However, she noted, “in contrast to pertuzumab, carboplatin clearly does add toxicity, and we don’t have evidence of a survival advantage for adding carboplatin in the metastatic setting.”
Conclusion
In conclusion, Drs. O’Regan and Zelnak emphasized that pathologic complete response is predictive of long-term outcomes only in subsets of early breast cancer, primarily triple-negative and HER2-positive/hormone receptor–negative patients. Especially in patients with low-grade estrogen receptor–positive tumors, it is not predictive. For patients in whom pCR is important, but is not achieved, more aggressive therapy and/or a clinical trial are warranted.
Finally, clinicians and patients should not consider pathologic complete response the be-all and end-all for treatment outcomes. “You can’t really look just at pCR,” Dr. Zelnak said, “as the final indication for how patients will do.” ■
Disclosure: Dr. O’Regan is a consultant for and received research support from Novartis and Genentech/Roche. Dr. Zelnak reported no potential conflicts of interest.
References
1. Cortazar P, Zhang L, Untch M, et al: Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 384:164-172, 2014.
2. Zelnack AB, Murali S, Styblo TM, et al: Phase II trial evaluating the use of 21-gene recurrence score to select preoperative therapy in hormone receptor-positive breast cancer. ASCO Annual Meeting. Abstract 562. Presented June 1, 2013.
3. Von Minckwitz G, Untch M, Blohmer JU, et al: Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796-1804, 2012.
4. Gianni L, Pienkowski T, Im YH, et al: Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13:25-32, 2012.
5. Schneeweiss A, Chia S, Hickish T, at al: Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24:2278-2284, 2013.
6. Swain SM, Kim SB, Cortés J, et al: Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 14:461-471, 2013.
7. Piccart-Gebhart M, Holmes AP, de Azambuja E, et al: The association between event-free survival and pathological complete response to neoadjuvant lapatinib, trastuzumab or their combination in HER2-positive breast cancer survival follow-up analysis of the NeoALTTO study (BIG 1-06). 2013 San Antonio Breast Cancer Symposium. Abstract S1-01. Presented December 11, 2013.
8. Piccart-Gebhart MJ, Holmes AP, Baselga J, et al: First results from the phase III ALTTO trial (BIG 2-06; NCCTG [Alliance] N063D) comparing one year of anti-HER2 therapy with lapatinib alone, trastuzumab alone, their sequence, or their combination in the adjuvant treatment of HER2-positive early breast cancer. 2014 ASCO Annual Meeting. Abstract LBA4. Presented June 1, 2014.
9. Carey L, Berry DA, Ollila D, et al: Clinical and translational results of CALGB 40601: A neoadjuvant phase III trial of weekly paclitaxel and trastuzumab with or without lapatinib for HER2-positive breast cancer. ASCO Annual Meeting. Abstract 500. Presented June 2, 2013.
10. Bhargava R, Dabbs DJ, Beriwal S, et al: Semiquantitative hormone receptor level influences response to trastuzumab-containing neoadjuvant chemotherapy in HER2-positive breast cancer. Mod Pathol 24:367-374, 2011.
11. Sikov WM, Berry DA, Perou CM, et al: Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant weekly paclitaxel followed by dose-dense AC on pathologic complete response rates in triple-negative breast cancer: CALGB 40603. 2013 San Antonio Breast Cancer Symposium. Abstract S5-01. Presented December 13, 2013.