Young women with early breast cancer may be more likely to resume menses and become pregnant when treated with a luteinizing hormone–releasing hormone (LH-RH) analog (also known as a gonadotropin-releasing hormone [GnRH] analog) along with chemotherapy, according to the final follow-up of PROMISE-GIM6, a phase III multicenter trial reported at the 2014 Breast Cancer Symposium.1
“Data from PROMISE and POEMS [another recent trial] support the use of LH-RH analogs during chemotherapy as a strategy to preserve ovarian function and fertility in breast cancer patients,” said Matteo Lambertini, MD, an oncology fellow working with Dr. Del Mastro at the IRCCS AOU San Martino-IST in Genova, Italy.
Historically Mixed Results
Eight randomized trials and eight systematic reviews have evaluated the role of LH-RH analogs as a strategy to preserve ovarian function in breast cancer patients, with mixed results. Data on long-term outcomes is scarce, he said.
The current ASCO and European Society for Medical Oncology (ESMO) guidelines consider this approach experimental. Dr. Lambertini suggested that “current guidelines should consider updating recommendations for fertility preservation in this setting, adding LH-RH analogs as a widely available option.”
While calling the PROMISE study “impressive” in its achievement of long-term outcome data on more than 85% of its population, Hope S. Rugo, MD, Professor of Medicine and Director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center, cautioned that the data on fertility preservation remain premature. “[LH-RH] analogs,” she said, “are not a substitute for established methods.”
PROMISE Details
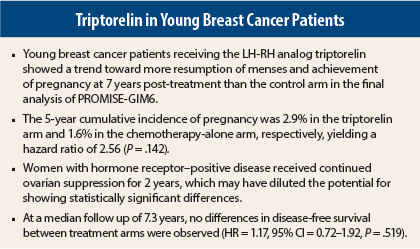
Dr. Lambertini presented updated results from PROMISE-GIM6 after 7.3 years of follow-up. The 1-year results showed a highly significant 72% reduction in the risk of treatment-related early menopause in women receiving adjuvant triptorelin (Trelstar), vs chemotherapy alone.2
In the updated analysis, women in the PROMISE-GIM6 study were more than twice as likely to become pregnant over 7 years when treated with adjuvant triptorelin compared with chemotherapy alone. The fact that disease-free survival was similar confirmed the safety of the combination in cancer patients, Dr. Lambertini said.
PROMISE-GIM6 was a multicenter, open-label trial of 281 premenopausal women with early-stage breast cancer who were candidates for neoadjuvant or adjuvant chemotherapy. Eighty percent of subjects had hormone receptor–positive disease.
Patients were randomly assigned to chemotherapy alone (n = 133) or chemotherapy plus triptorelin at 3.75 mg every 4 weeks (n = 148) starting at least 1 week prior to chemotherapy and continued for the duration of treatment.
After a median follow-up of 7.3 years, these were the key findings:
- There were eight pregnancies in the triptorelin arm vs three in the chemotherapy-alone arm. The incidence rate of pregnancy was 0.9 per 100 person-years vs 0.4 per 100 person-years in the respective arms.
- The 5-year cumulative incidence of pregnancy was 2.9% in the triptorelin arm and 1.6% in the chemotherapy-alone arm, respectively, yielding a hazard ratio (HR) of 2.56 (P = .142).
- The 5-year cumulative incidence estimate of resumption of menses at any time was 72.6% vs 64.0%, respectively (HR = 1.28, P = .071).
- The 5-year disease-free survival rate was 80.5% and 83.7%, respectively (HR = 1.17, P = .519).
- The 5-year overall survival was 93.3%, with no statistical comparison between the arms due to a low number of events.
Dr. Lambertini said that while these findings were nonsignificant trends, they support those from the recent POEMS study, reported at the 2014 ASCO Annual Meeting.3 In POEMS, hormone receptor–negative patients receiving the LH-RH agonist goserelin (Zoladex) had a lower rate of ovarian failure at 2 years (8% vs 22%) and twice the likelihood of pregnancy (adjusted odds ratio = 2.45) compared with women not receiving this drug. Unlike PROMISE, the study did not include women with hormone receptor–positive disease.
Caution: More Data Needed
In her discussion of this paper, Dr. Rugo emphasized that fertility preservation is a factor for many of the 20,000 women of childbearing age diagnosed with breast cancer each year. “This is an important issue for oncologists and patients,” she said.
The most important risk factor for ovarian failure, she noted, is increasing age, followed by diminished ovarian reserve (which is difficult to measure). Type, dose, and schedule of chemotherapy also matter (higher doses are related to greater risk), as does the use of endocrine therapy.
Options for preserving fertility include ovarian stimulation, ie, cryopreservation of embryos and oocytes. “Ovarian stimulation is the most proven method of fertility preservation for young women with breast cancer, and with current techniques, this now takes only about 2 weeks, which is a very modest delay for most women starting chemotherapy,” she noted.
On the other hand, the use of LH-RH agonists as a means of ovarian suppression for fertility preservation has mostly been studied in small trials, with mixed results and no long-term data. This strategy has also not been met with enthusiasm by some fertility specialists, who point out that it may not be protecting the primordial follicles. Alkylating agents induce amenorrhea by affecting the resting oocyte/primordial follicles, she indicated.
In the PROMISE study, the impact of triptorelin is hard to determine, since patients whose ovarian function resumed within 1 year could receive monthly triptorelin for at least 2 years, said Dr. Rugo. Also, 93% received adjuvant endocrine therapy, 23% with tamoxifen alone and almost 70% with tamoxifen or an aromatase inhibitor plus triptorelin.
“The investigators did this because they believed that ovarian suppression was an important part of treating patients with estrogen receptor–positive disease,” she noted. Because of this design, long-term follow-up understandably showed no statistically significant difference in the resumption of menses 7 years later, she said.
“I would also be hesitant for patients with higher-risk estrogen receptor–positive breast cancer to preserve their ovaries and ovarian function, since various datasets suggest that ovarian function suppression may be an important part of treatment, as it improves disease-free survival,” she said. “Maybe we don’t want all of the ovarian function to improve right away, following treatment in estrogen receptor–positive patients with high-risk disease.”
The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-30 substudy established that in women with high-risk estrogen receptor–positive disease, amenorrhea for ≥ 6 months predicted for improved overall survival (HR = 0.52, P = .002) and disease-free survival (HR = 0.51, P < .001).4
There are also caveats to the findings from POEMs, which was compromised by the fact that for almost half its enrollees, long-term outcomes could not be determined, she added.
Strategies for reducing ovarian failure, she added, are hard to assess, as fertility is not the same as amenorrhea, levels of follicle-stimulating hormone are not predictive, and the desire for pregnancy is highly individualized and changes over time. Furthermore, definitions of ovarian failure vary widely, and modern treatments may be associated with lower rates than are currently estimated from older regimens, she pointed out.
Conclusions
In short, Dr. Rugo cautioned, “The use of [LH-RH] agonists during chemotherapy to protect ovarian function remains controversial. Given the lack of apparent risk, however, they could be considered in patients with estrogen receptor–negative disease, with a discussion of the known benefits. They are not a substitute for established methods.”
Ongoing trials (eg, SOFT) and future trials (eg, POISE) are expected to provide more answers on this issue. ■
Disclosure: Dr. Lambertini reported no potential conflicts of interest.
References
1. Lambertini M, Boni L, Michelotti A, et al: Long-term outcome results of the phase III PROMISE-GIM6 study evaluating the role of LHRH analog during chemotherapy as a strategy to reduce ovarian failure in early breast cancer patients. ASCO Breast Cancer Symposium. Abstract 105. Presented September 4, 2014.
2. Del Mastro L, Boni L, Michelotti A, et al: Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: A randomized trial. JAMA 306:269-276, 2011.
3. Moore HCF, Unger JM, Phillips KA, et al: Phase III trial (Prevention of Early Menopause Study [POEMS]-SWOG S0230) of LHRH analog during chemotherapy to reduce ovarian failure in early-stage, hormone receptor-negative breast cancer. ASCO Annual Meeting. Abstract LBA505. Presented May 31, 2014.
4. Swain SM, Jeong JH, Geyer CE Jr, et al: Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med 362:2053-2065. 2010.