Ipilimumab (Yervoy) is a fully humanized immunoglobulin (Ig)G1 monoclonal antibody that binds to and inhibits cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4). It acts as a T-cell potentiator, leading to increases in T-cell activation and interleukin-2 secretion. Ipilimumab is U.S. Food and Drug Administration–approved for the treatment of late-stage (metastatic or unresectable) melanoma and is the first drug to demonstrate a survival benefit in the setting of metastatic melanoma.
Ipilimumab-Related Adverse Events
Treatment with ipilimumab is associated with numerous immune-related adverse events, including enterocolitis, hepatitis, hypophysitis, uveitis, and skin-related side effects. Skin-related adverse events include rashes, pruritus, and vitiligo. The overall incidence of all-grade rash and high-grade rash is 24% and 2%, respectively.
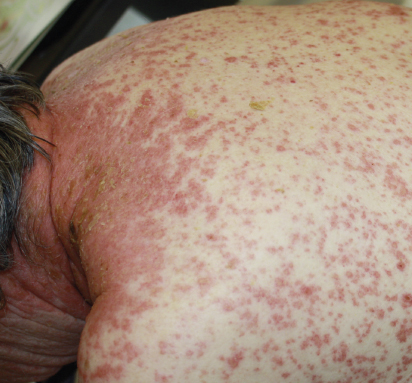
The ipilimumab-associated cutaneous eruption is characterized by discrete, erythematous, minimally scaly, pruritic papules that can coalesce into thin plaques, most often involving the trunk and extremities (Fig. 1). The face and head are less frequently involved, and palms and soles are usually spared.
The rash usually occurs early in the course of treatment, in the first 3 to 4 weeks after the first dose; however, rash development earlier or even after completion of therapy is possible. The rash can worsen with each ipilimumab dose and can be associated with a significant increase in peripheral eosinophilia.
Histologically, epidermal spongiosis, papillary dermal edema, and a perivascular lymphocytic infiltrate with eosinophils can be seen. The risk of rash associated with ipilimumab does not appear to be dose-dependent. Usually well tolerated, the cutaneous eruption is often self-limited, but can take weeks to months to resolve.
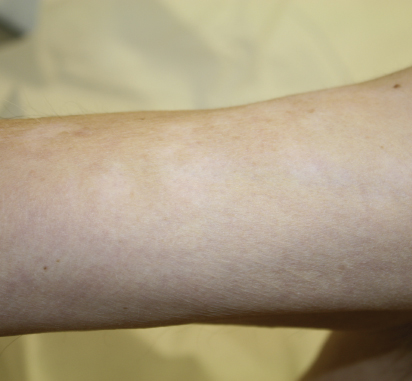
Pruritus, or itch, has been reported to occur in up to 31% of patients on ipilimumab. Pruritus can be associated with both rashes and dry skin, but may also occur separately as a direct result of enhanced immune system activation in the skin. Vitiligo, or depigmentation of the skin, can also develop as a result of ipilimumab treatment, but has been reported in only 2% to 4% of patients (Fig. 2). Such depigmentation tends to be irreversible and can be limited or widespread.
Treatment Recommendations
For grades 1 and 2 rashes, treatment consists of topical corticosteroids. For grade 3 rashes, oral corticosteroids may be required, such as oral prednisone at 1 mg/kg daily, while ipilimumab is held until rash improvement. For grade 4 rashes, oral corticosteroids at higher doses, such as oral prednisone at 1 to 2 mg/kg daily, may be required, and permanent discontinuation of ipilimumab must be considered.
For pruritus, general recommendations include use of alcohol-free emollient creams to moisturize the skin. Cold compresses, oatmeal baths, and topical corticosteroids may be helpful in relieving pruritus. Systemic antihistamines can also be beneficial. Nonsedating second-generation antihistamines, such as loratadine, cetirizine, and fexofenadine are recommended for daytime treatment, whereas first-generation, sedating antihistamines, such as diphenhydramine and hydroxyzine, are generally recommended for nighttime use. Doxepin, a tricyclic antidepressant, has also been used successfully in reducing pruritus, and is available in both topical and oral preparations.
Special caution should be taken when prescribing antihistamines and antidepressants to patients with cancer—particularly in the elderly—to avoid oversedation. Other agents, including GABA agonists (eg, gabapentin) and aprepitant (neurokinin-1 receptor antagonist), are being investigated as potential antipruritic agents.
While there is no known treatment for ipilimumab-induced vitiligo, prevention of photosensitivity or sunburn in depigmented areas is important. Patients should be advised to use a broad-spectrum sunscreen of SPF 30, applied every 2 hours to sun-exposed areas, in addition to wearing sun-protective clothing, sunglasses, and broad-rimmed hats. Additionally, avoidance of direct sun exposure between 10:00 AM and 4:00 PM is recommended.
Recognition and treatment of ipilimumab-induced cutaneous reactions are important, as they are a common manifestation of immune-related side effects. Proper management can help in maintaining a patient’s treatment with ipilimumab. ■
Disclosure: Dr. Choi reported no potential conflicts of interest.
GUEST EDITOR
Dermatologic Events in Oncology is guest edited by Mario E. Lacouture, MD, an Associate Member in the Division of Dermatology, Department of Medicine, at Memorial Sloan-Kettering Cancer Center, New York. He is a board-certified dermatologist with a special interest in dermatologic conditions that result from cancer treatments.