 Marie E. Wood, MD, of the Familial Cancer Program at the University of Vermont, Burlington, addressed clinically relevant issues in supportive care and survivorship at the Best of ASCO® Annual Meeting ‘11 in Miami.
Marie E. Wood, MD, of the Familial Cancer Program at the University of Vermont, Burlington, addressed clinically relevant issues in supportive care and survivorship at the Best of ASCO® Annual Meeting ‘11 in Miami.
Delayed Nausea and Vomiting
Two studies addressed the problem of chemotherapy-related delayed nausea and vomiting. In the large University of Rochester Community Clinical Oncology Program study (N = 1,021), 55% of patients experienced delayed nausea and 16% had delayed vomiting. In a comparison of four preventive approaches, palonosetron was no more effective than granisetron when both antiemetics were provided with dexamethasone on day 1 of chemotherapy and with prochlorperazine on days 2 and 3.1
Patients receiving dexamethasone on days 2 and 3 (in the comparison of dexamethasone vs no dexamethasone on these days) had significantly less delayed nausea but no reduction in delayed vomiting. Patients receiving aprepitant (Emend)/dexamethasone had significantly less delayed vomiting but no benefit over prochlorperazine in controlling nausea. The group receiving aprepitant plus palonosetron plus dexamethasone on day 1 and aprepitant/dexamethasone on days 2 and 3 had the lowest rate of delayed vomiting (8% vs 14%–24% in other groups).
 “This was a large study that included many groups and comparisons, though it did not study aprepitant alone,” Dr. Wood noted, indicating that such data would help determine how best to use this agent. The study also raised doubts that palonosetron is more effective than other 5HT3 receptor antagonists when part of a combination regimen, she added.
“This was a large study that included many groups and comparisons, though it did not study aprepitant alone,” Dr. Wood noted, indicating that such data would help determine how best to use this agent. The study also raised doubts that palonosetron is more effective than other 5HT3 receptor antagonists when part of a combination regimen, she added.
A second randomized phase III study of 68 germ cell tumor patients receiving a 5-day cisplatin-based regimen found that aprepitant given with a 5HT3 receptor antagonist plus dexamethasone increased the complete response rate (no vomiting, no rescue medication).2 Aprepitant was started at 125 mg on day 3, with 80 mg given on days 4 to 7. Complete responses were observed in 42% of the aprepitant arm vs 13% of controls (5HT3 receptor antagonist/dexamethasone; P < .0001).
ASCO guidelines, which recommend aprepitant/dexamethasone for patients receiving high–emetic risk agents, are largely based on consensus. “But now we have more level 1 evidence that aprepitant/dexamethasone does improve delayed nausea and vomiting,” Dr. Wood said. “The results may challenge how we think about and potentially treat delayed nausea and vomiting, in that we use aprepitant starting on day 3 of multiday cisplatin-based chemotherapy.… Unfortunately, no matter how you cut it, delayed nausea remains a significant problem.”
Thromboprophylaxis
A study of 3,212 cancer patients demonstrated the benefit of thromboprophylaxis with a novel ultra-low-molecular-weight heparin with high anti–factor Xa and residual anti–factor IIa activities.3 With subcutaneous semuloparin (20 mg/d), the rate of venous thromboembolism was reduced by 64%, from 3.4% with placebo to 1.2% (P < .0001), with very low rates of major or clinically relevant bleeding.
“Prophylaxis reduces the thrombosis risk in cancer patients by 50% [64% in this study], but it does not change survival,” Dr. Wood noted, adding that cost (high copays) is an issue, as is quality of life, due to the need for daily injections.
Prophylaxis may be reasonable based on individual risk, she said. Most risk is related to site: stomach, pancreas, and brain carry the highest risk, while breast, colorectal, and head and neck cancer have the lowest. Other risk factors are prechemotherapy platelet count > 350 × 109/L, hemoglobin < 10 g/dL, white cell count > 11 × 109/L, and body mass index > 35.
A published predictive model based on risk factors can determine 6-month risk of venous thromboembolism.4 “It may be reasonable to think about thromboprophylaxis in patients with more than a 10% risk,” she said.
Yoga’s Benefits Documented
In the first study of yoga to include a control group, regular practice of yoga was able to buffer the effects of radiotherapy on both patient-reported and biologic endpoints.5 The benefits were more than “stretching and social support,” the authors concluded.
The study randomized 178 breast cancer patients undergoing radiotherapy to yoga or stretching three times a week for 6 weeks during treatment, or to a waitlist control group. Fatigue was diminished in the yoga and stretch groups, whereas it increased among waitlist controls (P < .05). Yoga also significantly improved physical functioning (as assessed by the SF‑36 health survey) compared with controls, and was associated with significantly higher general health scores, steeper cortisol slope, and greater heart rate variability vs the control or stretch groups.
“There is mounting support for the importance of yoga and other integrated therapy in the care of cancer patients,” Dr. Wood commented.
Survivorship Plans
Survivorship care plans are advocated by the Institute of Medicine, but do they really improve health outcomes? Canadian researchers concluded that the survivorship care plan adopted by their centers had no impact on patient-reported outcomes or adherence to follow-up guidelines.6
All 408 early breast cancer patients were transitioning from oncology care to their primary care physician. One group received a formal survivorship care plan, which consisted of a treatment summary, patient version of follow-up guidelines, and local supportive care resources, reviewed with the patient by a nurse. Their primary care physician received copies of these documents, guidelines, and a reminder table of recommended follow-up visits and tests. The control group had a discharge visit and their physicians received a discharge letter, according to usual practice.
At 12 months, the intervention group demonstrated no additional improvement in psychosocial adjustment (the primary endpoint) or any of the secondary outcomes (continuity of care, health-related quality of life [SF-36], patient satisfaction, and guideline adherence).
“This was a different result than the investigators had hypothesized,” she noted. However, the study did prove that patients can be successfully transferred to a primary care physician; less than 10% in each arm returned to their oncologist for care.
Brief Screening Tool
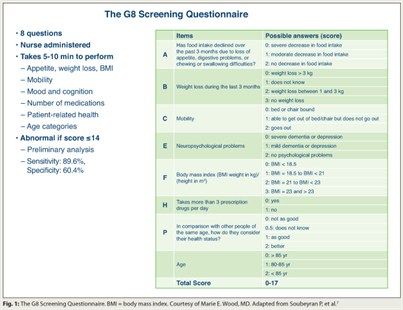 The comprehensive geriatric assessment (CGA) is an appropriate but time-consuming method of predicting tolerance to cancer treatment in older patients.7 French investigators reported that the brief G8 assessment (Fig. 1) was more sensitive than the Vulnerable Elders Survey (VES)-13, a 13-item function-based self-report questionnaire, in predicting an abnormal CGA.
The comprehensive geriatric assessment (CGA) is an appropriate but time-consuming method of predicting tolerance to cancer treatment in older patients.7 French investigators reported that the brief G8 assessment (Fig. 1) was more sensitive than the Vulnerable Elders Survey (VES)-13, a 13-item function-based self-report questionnaire, in predicting an abnormal CGA.
The ONCODAGE project validated the G8 among 1,425 patients with a variety of cancer types. Overall, abnormal test rates on the CGA, G8, and VES-13 were, respectively, 80.1%, 68.4%, and 60.1%. The G8 screening tool was more sensitive than the VES-13 though equivalent in other parameters. Both brief screening tests were administered in less than 5 minutes, whereas the mean time for the CGA was 67 minutes.
“An abbreviated tool is good at picking up patients who may benefit from a more thorough evaluation (CGA) or collaborative care,” she said, reminding physicians that “geriatric assessment that screens for vulnerability actually predicts survival in cancer patients.” ■
Disclosure: Dr. Wood reported no potential conflicts of interest. Dr. Hayes holds stock in Oncimmune, LLC, has served as a consultant for Chugai Pharmaceuticals and Biomarker Strategies, and has received research funding from Pfizer, Novartis, and Veridex (Johnson & Johnson).
SIDEBAR: Who Should Manage Survivorship Care?
References
1. Morrow GR, Roscoe JA, Heckler C, et al: A phase III study for prevention of delayed nausea. 2011 ASCO Annual Meeting. Abstract 9012. Presented June 6, 2011.
2. Brames MJ, Picus J, Yu M, et al: Phase III, double-blind, placebo-controlled, crossover study evaluating a 5HT3 antagonist plus dexamethasone with or without aprepitant in patients with germ cell tumor receiving 5-day cisplatin combination chemotherapy. 2011 ASCO Annual Meeting. Abstract 9013. Presented June 6, 2011.
3. Agnelli G, George DJ, Fisher W, et al: The ultra-low molecular weight heparin semuloparin for prevention of venous thromboembolism in patients with cancer receiving chemotherapy. 2011 ASCO Annual Meeting. Abstract LBA9014. Presented June 6, 2011.
4. Khorana AA, Kuderer NM, Culakova E, et al: Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 111:4902-4907, 2008.
5. Cohen L, Chandwani K, Raghuram NV, et al: Effect of yoga on quality of life, cortisol rhythm, and heart rate variability for women with breast cancer undergoing radiotherapy. 2011 ASCO Annual Meeting. Abstract 9009. Presented June 6, 2011.
6. Grunfeld E, Levine MN, Julian JA, et al: Results of a multicenter randomized trial to evaluate a survivorship care plan for breast cancer survivors. 2011 ASCO Annual Meeting. Abstract 9005. Presented June 4, 2011.
7. Soubeyran P, Bellera C, Goyard J, et al: Validation of the G8 screening tool in geriatric oncology. 2011 ASCO Annual Meeting. Abstract 9001. Presented June 5, 2011.

 It has been suggested that after completing their treatment, cancer patients can be transitioned to primary care providers for continued “survivorship” care. But at the Best of ASCO meeting, speakers and audience members alike felt survivorship care is the domain of the treating oncologist.
It has been suggested that after completing their treatment, cancer patients can be transitioned to primary care providers for continued “survivorship” care. But at the Best of ASCO meeting, speakers and audience members alike felt survivorship care is the domain of the treating oncologist.