Indication—Ipilimumab (Yervoy) is indicated for the treatment of unresectable or metastatic melanoma. The pivotal trial of ipilimumab, performed in patients who had received at least one prior treatment with interleukin-2 (Proleukin), dacarbazine, temozolomide, fotemustine, or carboplatin, assessed ipilimumab with or without an experimental gp100 vaccine vs the gp100 vaccine alone. Patients receiving ipilimumab alone had a 34% reduction in risk for death (HR = 0.66, P = .0026), and those receiving ipilimumab plus gp100 vaccine had a 32% reduction in risk (HR 0.68, P = .0004) compared with patients receiving the gp100 vaccine alone. Median survival durations were 10 months in the two ipilimumab groups and 6 months in the gp100 group.
How it works—Cytotoxic T lymphocytes (CTLs) are immune cells that kill such target cells as those infected with virus or intracellular bacteria or protozoa and cancer cells. Ipilimumab is a fully human antibody that binds to the cytotoxic T lymphocyte–associated antigen 4 (CTLA-4), a molecule on the surface of cytotoxic T cells that is an important negative regulator of T-cell response. By blocking CTLA-4, ipilimumab acts to sustain cytotoxic T lymphocyte activity by augmenting T-cell activation and proliferation. The immune-related adverse effects of ipilimumab are the result of this sustained/augmented activity.
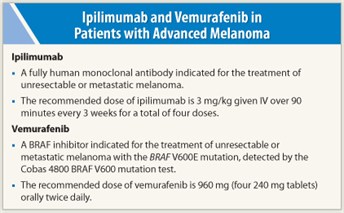
How it is given—The recommended dose of ipilimumab is 3 mg/kg given IV over 90 minutes every 3 weeks for a total of four doses.
Scheduled doses of ipilimumab should be withheld for any moderate immune-related reactions or symptomatic endocrinopathy. For patients with complete or partial resolution of adverse reactions (grade 0 or 1) who are receiving < 7.5 mg of prednisone (or equivalent) per day, ipilimumab can be resumed at this dosing schedule until the earlier of administration of all four planned doses or 16 weeks from the first dose.
Ipilimumab should be permanently discontinued for persistent moderate adverse reactions or inability to reduce prednisone dose to 7.5 mg/d; failure to complete the treatment course within 16 weeks from the first dose; and severe or life-threatening adverse reactions, including any of the following: (a) colitis with abdominal pain, fever, ileus, or peritoneal signs; increase in stool frequency (7 or more over baseline), stool incontinence, need for IV hydration for > 24 hours, or gastrointestinal hemorrhage or perforation; (b) AST or ALT > 5 times the upper limit of normal or total bilirubin > 3 times upper limit of normal; (c) Stevens-Johnson syndrome, toxic epidermal necrolysis, or rash complicated by full thickness dermal ulceration, or necrotic, bullous, or hemorrhagic manifestations; (d) severe motor or sensory neuropathy, Guillain-Barré syndrome, or myasthenia gravis; (e) severe immune-mediated reactions involving any organ system (eg, nephritis, pneumonitis, pancreatitis, noninfectious myocarditis); and (f) immune-mediated ocular disease that is unresponsive to topical immunosuppressive therapy.
Safety profile—Ipilimumab has a black box warning for immune-mediated adverse reactions. These reactions may affect any organ system and include enterocolitis, hepatitis, dermatitis (including toxic epidermal necrolysis), neuropathy, and endocrinopathy. Although the majority of these reactions were observed to have onset during treatment, some occurred weeks to months after discontinuation of ipilimumab. Ipilimumab should be discontinued and systemic high-dose corticosteroid therapy initiated for any severe immune-mediated reaction.
 In the pivotal clinical trial of ipilimumab, the most common adverse reactions were fatigue, diarrhea, pruritus, rash, and colitis. Severe or fatal immune-related reactions (grade 3-5) included enterocolitis in 7% of patients, dermatitis in 2.5%, hepatotoxicity in 2%, and endocrinopathies in 1.8% (all of these patients had hypopituitarism and some had such concomitant endocrinopathies as adrenal insufficiency, hypogonadism, and hypothyroidism). One case of fatal Guillain-Barré syndrome and one case of severe peripheral motor neuropathy were reported in this study, and myasthenia gravis and additional cases of Guillain-Barré syndrome have been reported in other experience in the ipilimumab clinical development program.
In the pivotal clinical trial of ipilimumab, the most common adverse reactions were fatigue, diarrhea, pruritus, rash, and colitis. Severe or fatal immune-related reactions (grade 3-5) included enterocolitis in 7% of patients, dermatitis in 2.5%, hepatotoxicity in 2%, and endocrinopathies in 1.8% (all of these patients had hypopituitarism and some had such concomitant endocrinopathies as adrenal insufficiency, hypogonadism, and hypothyroidism). One case of fatal Guillain-Barré syndrome and one case of severe peripheral motor neuropathy were reported in this study, and myasthenia gravis and additional cases of Guillain-Barré syndrome have been reported in other experience in the ipilimumab clinical development program.
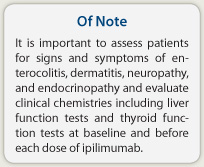
It is important to assess patients for signs and symptoms of enterocolitis, dermatitis, neuropathy, and endocrinopathy and evaluate clinical chemistries including liver function tests and thyroid function tests at baseline and before each dose of ipilimumab.
Suggested Readings
1. Robert C, Thomas L, Bondarenko I, et al: Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364:2517-2526, 2011.
2. Hodi FS, O’Day SJ, McDermott DF, et al: Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711-723, 2010.
3. Wolchok JD, Thomas L, Bondarenko I, et al: Phase III randomized study of ipilimumab plus dacarbazine vs DTIC alone as first-line treatment in patients with unresectable stage II or IV melanoma. 2011 ASCO Annual Meeting. Abstract LBA5. Presented June 5, 2011.
Vemurafenib
Indication—Vemurafenib (Zelboraf) is indicated for the treatment of patients with unresectable or metastatic melanoma with the BRAF V600E mutation as detected by an FDA-approved test. It is not recommended for use in patients with wild-type BRAF. In the clinical trial supporting approval of vemurafenib, performed in treatment-naive patients with the V600E mutation, vemurafenib treatment reduced risk of mortality by 56% (HR = 0.44, P < .0001) and reduced risk of progression by 74% (HR = 0.26, P < .0001) compared with dacarbazine. Median survival durations were not reached in the vemurafenib group vs 7.9 months in the dacarbazine group and median progression-free survival durations were 5.3 and 1.6 months, respectively.
Confirmation of the BRAF V600E mutation using an FDA-approved test is required for vemurafenib treatment. All patients in the pivotal clinical trial had positive results on the cobas® 4800 BRAF V600 Mutation Test.
How it works—Vemurafenib is a low-molecular weight, orally available inhibitor of some mutated forms of the BRAF serine threonine kinase. This enzyme is involved in the transduction of mitogenic signals from the cell membrane to the nucleus. Some mutations in the BRAF gene, including V600E (valine replaced by glutamic acid), result in constitutively activated BRAF proteins, which can cause cell proliferation in the absence of the growth factors normally required for proliferation. By inhibiting this enzyme, vemurafenib interrupts the BRAF/MEK step of the BRAF/MEK/ERK pathway and causes programmed cell death in melanoma cells. Approximately one-half of melanomas have the BRAF V600E mutation.
How it is given—The recommended dose of vemurafenib is 960 mg (four 240 mg tablets) orally twice daily. Doses are taken approximately 12 hours apart with or without a meal. The tablets are swallowed whole with water.
Dose reductions or interruption or discontinuation of treatment may be required for symptomatic adverse effects or QT interval prolongation. For intolerable grade 2 or grade 3 adverse events, it is recommended that treatment be interrupted until resolution (grade 0 or 1) and resumed at 720 mg twice daily and 480 mg twice daily after the first and second occurrences, respectively, with treatment discontinued for a third occurrence. For grade 4 adverse events, treatment should be discontinued or interrupted until resolution and then resumed at 480 mg twice daily for a first occurrence; in patients resuming treatment, treatment should be discontinued after a second occurrence. Dose reductions to lower than 480 mg twice daily are not recommended.
 Safety profile—In two clinical studies providing safety data (one in treatment-naive patients and one in previously treated patients), the most common adverse reactions to vemurafenib were arthralgia, rash, alopecia, fatigue, photosensitivity reaction, nausea, pruritus, and skin papilloma. The most common grade 3 adverse events were cutaneous squamous cell carcinoma and rash. Grade 4 adverse events occurred in < 4% of patients in both studies.
Safety profile—In two clinical studies providing safety data (one in treatment-naive patients and one in previously treated patients), the most common adverse reactions to vemurafenib were arthralgia, rash, alopecia, fatigue, photosensitivity reaction, nausea, pruritus, and skin papilloma. The most common grade 3 adverse events were cutaneous squamous cell carcinoma and rash. Grade 4 adverse events occurred in < 4% of patients in both studies.
The following adverse reactions warrant warnings/precautions.
- Cutaneous squamous cell carcinoma occurred in 24% of patients and new primary malignant melanomas were observed. These should be managed by excision and treatment continued without dose adjustment. Patients should undergo dermatologic evaluation prior to the start of treatment and every 2 months during treatment.
- Serious hypersensitivity reactions, including anaphylaxis, were seen during and at reinitiation of treatment and severe dermatologic reactions were reported, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Treatment should be discontinued in patients with serious hypersensitivity reactions and severe dermatologic reactions.
- QT prolongation was documented in a phase II QT substudy. ECG and electrolytes should be monitored before treatment and after any dose modification, and ECGs should be monitored on day 15, monthly during the first 3 months of treatment, and every 3 months thereafter. Vemurafenib treatment should be interrupted if the QTc exceeds 500 ms, with correction of electrolyte abnormalities other cardiac risk factors for QT prolongation.
- Since photosensitivity has been reported, patients should be advised to avoid sunlight during vemurafenib treatment.
- Liver enzymes and bilirubin should be measured before and monthly during treatment for liver function abnormalities.
- Since serious ophthalmologic reactions, including uveitis, iritis, and retinal vein occlusion, were observed, patients should be regularly monitored for ophthalmologic changes.
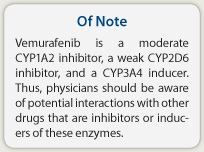
Vemurafenib is a moderate CYP1A2 inhibitor, a weak CYP2D6 inhibitor, and a CYP3A4 inducer. Thus, physicians should be aware of potential interactions with other drugs that are inhibitors or inducers of these enzymes. ■
Suggested Readings
1. Chapman PB, Hauschild A, Robert C, et al: Phase III randomized, open-label, multicenter trial (BRIM3) comparing BRAF inhibitor vemurafenib with dacarbazine in patients with V600E BRAF-mutated melanoma. 2011 ASCO Annual Meeting. Abstract LBA4. Presented June 5, 2011.
2. Ribas A, Kim KB, Schucter LM, et al: BRIM-2: An open-label, multicenter phase II study of vemurafenib in previously treated patients with BRAF V600E mutation-positiove metastatic melanoma. 2011 ASCO Annual Meeting. Abstract 8509. Presented June 4, 2011.