Treatment and prevention of bone metastases in patients with prostate cancer is coming of age, according to several studies presented at the European Multidisciplinary Cancer Congress (ECCO/ESMO/ESTRO). Among the most impressive studies reported was an international phase III trial of radium-223, an investigational radiopharmaceutical, evaluated in men with castration-resistant prostate cancer and symptomatic bone metastases.  For the first time, a survival advantage was seen in the treatment arm receiving radium-223 along with delayed time to first skeletal-related event (Fig. 1).1
For the first time, a survival advantage was seen in the treatment arm receiving radium-223 along with delayed time to first skeletal-related event (Fig. 1).1
A separate phase III study demonstrated that denosumab (Prolia, Xgeva) delayed the development of bone metastasis in men with prostate cancer,2 and a third study found that ibandronate was as effective as radiotherapy in treating pain related to bone metastasis in prostate cancer.3
Radium-223
“Radium-223 chloride improved survival and time to [skeletal-related events] and was very well tolerated. If approved, this drug is likely to become standard of care,” said Chris Parker, MD, Royal Marsden Hospital, London. “This is the first drug targeted to bone metastases in prostate cancer that has been shown to improve survival. Other 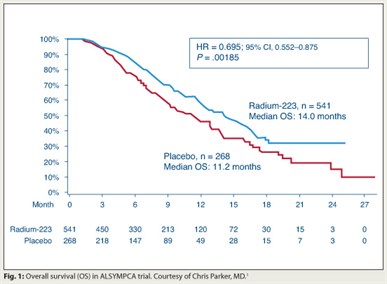 drugs targeted to the bone treat symptoms,” he added.
drugs targeted to the bone treat symptoms,” he added.
The ALSYMPCA international phase III trial (ALpharadin [radium-223] in SYMptomatic Prostate Cancer) was selected as a Late-Breaking Abstract for the Presidential Session at the European Congress.
Injectable radium-223 targets areas of new bone formation, such as cancer metastasis, and emits highly damaging short-range alpha particles. This kills the tumor cells but spares the more distant cells in the bone marrow, Dr. Parker explained.
“Only a few hits are required with radium-223, whereas with beta radiation [used in other radiopharmaceuticals], thousands of hits are needed, and there is damage to the surrounding tissue,” Dr. Parker said. Treatment with radium-223 takes 5 minutes and is performed on an outpatient basis.
Randomized Study Design
 The ALSYMPCA international trial enrolled 922 men from 19 countries with hormone-refractory prostate cancer and multiple symptomatic bone metastases. Patients were randomized 2:1 to either 6 injections of radium-223 given 1 out of every 4 weeks or sham placebo injections; all patients also received current standard of care.
The ALSYMPCA international trial enrolled 922 men from 19 countries with hormone-refractory prostate cancer and multiple symptomatic bone metastases. Patients were randomized 2:1 to either 6 injections of radium-223 given 1 out of every 4 weeks or sham placebo injections; all patients also received current standard of care.
Median overall survival was 14 months in the radium-223 arm vs 11.2 months in placebo patients (P= .000185). Median time to first skeletal-related event was 13.6 months vs 8.4 months, respectively (P = .00046), representing a 39% improvement with the alpha-emitting agent. Other findings were normalization of alkaline phosphatase in 33% of men treated with radium-223 versus 1% of placebo patients, and a 49% improvement in time to PSA progression (P = .00015).
Dr. Parker said that radium-223 was well tolerated. “This is one of the very few trials with more adverse events reported in the placebo group,” he said. Grades 3 and 4 hematologic toxicities were: anemia, 11% with radium-223 vs 12% with placebo; neutropenia, 2% vs 1%, respectively; and thrombocytopenia, 4% vs 2%, respectively. The most common nonhematologic adverse events in both groups were bone pain, nausea, diarrhea, constipation, and vomiting.
Quality-of-life data were collected and will be analyzed at a future time, Dr. Parker said.
Denosumab
Denosumab delayed the onset of bone metastasis by 4 months compared with placebo in men with castration resistant prostate cancer in a separate international, double-blind, randomized trial.2 The main results were reported at the May 2011 meeting of the American Urological Association. Stephane Oudard, MD, George Pompidou Hospital, Paris, France, reported subgroup analyses at the European Congress showing that denosumab’s effects were  consistent across all prespecified subgroups.
consistent across all prespecified subgroups.
“This is the first demonstration that targeting the bone environment prevents bone metastasis,” said J. Bellmunt, MD, Hospital del Mar, Barcelona, Spain, formal discussant of the subgroup analyses.
“A caveat for this trial is that overall progression-free survival was not altered by denosumab. Based on current data, overall survival was identical compared with placebo. This may not meet regulatory muster. The FDA may not consider bone scan changes an important endpoint,” stated Oliver Sartor, MD, Director of the Prostate Program at Tulane Cancer Center in New Orleans.
Ibandronate
A late-breaking abstract presented at the European Congress showed that single-dose ibandronate was no better than a single dose of radiation therapy for localized metastatic bone pain in the multicenter randomized RIB trial.3 That study included 407 men with prostate cancer and metastatic bone pain who were randomized to either treatment with ibandronate or radiation therapy; crossover was allowed at 4 weeks if pain relief was insufficient. Overall survival was no different between the two treatment arms. The study was presented by Peter J. Hoskin, MD, Mt. Vernon Cancer Center, Northwood, Middlesex, UK.
Discussant of this abstract, Daniel Zips, MD, Technische Universität, Dresden, Germany, said, “This is the first large randomized controlled trial to compare radiotherapy vs bisphosphonates in the treatment of metastatic bone pain. The major finding was equivalence for pain relief. However, with both treatments, 50% of patients don’t have a good response. We need better therapies for bone pain.”
Dr. Zips said that in his view, radiotherapy will remain the standard of care for metastatic bone pain. “However, ibandronate remains an effective treatment option for patients who can’t tolerate radiotherapy,” Dr. Zips added. ■
Disclosure: Dr. Parker has received honoraria from Bayer for advisory boards. Dr. Sartor has been a consultant to Amgen.
Expert Point of View: International Prostate Cancer Studies Report Inroads in Managing Bone Metastases
References
1. Parker C, Heinrich D, O’Sullivan JM, et al: Overall survival benefit of radium-223 chloride (Alpharadin) in the treatment of patients with symptomatic bone metastases in castration-resistant prostate cancer: A phase III randomized trial (ALSYMPCA). Eur J Cancer 47 (suppl 2): 3 , 2011 (Abstract 1LBA).
2. Oudard S, Smith M, Karsh L, et al: Denosumab and bone metastasis-free survival in men with castrate-resistant prostate cancer: Subgroup analyses from an international, double-blind, randomized, phase 3 trial. Eur J Cancer 47 (suppl 1): S484, 2011 (Abstract 7003).
3. Hoskin P, Sundar S, Reczko K, et al: A multicenter randomized trial of ibandronate compared to single dose radiotherapy for localized metastatic bone pain in prostate cancer (RIB). Eur J Cancer 47 (suppl 2): 6, 2011 (Abstract 7LBA).

 "Radium-223 chloride is an effective, well tolerated, and convenient treatment, and it has a survival benefit. These favorable characteristics may well promote its use in clinical practice,” said formal discussant of this abstract, Wim J.G. Oyen, MD, Radboud University Nijmegen Medical Center,...
"Radium-223 chloride is an effective, well tolerated, and convenient treatment, and it has a survival benefit. These favorable characteristics may well promote its use in clinical practice,” said formal discussant of this abstract, Wim J.G. Oyen, MD, Radboud University Nijmegen Medical Center,...
